Rotavirus is the most common cause of severe gastroenteritis in children worldwide. In Australia during the 20th century, it was a major cause of infant death. NHMRC-funded researchers at The Royal Children’s Hospital, Melbourne (RCH) and the University of Melbourne discovered rotavirus. Along with researchers at Murdoch Children’s Research Institute (MCRI) they made important contributions to the development of vaccines against it, leading to significant decreases in hospitalisation for infant diarrhoea globally.
Origin
Gastroenteritis is an illness triggered by infection and inflammation of the digestive system. Typical symptoms include severe watery diarrhoea, vomiting, fever and abdominal pain. In many cases the condition heals itself within a few days. However, when experienced by infants and when constant fluid and electrolyte loss occurs, it can be lethal.
Infant diarrhoea has been a significant problem throughout recorded history. In Australia during the first decade of the 20th century it accounted for one quarter of all deaths of children aged 0–4 and ranked second only to tuberculosis as a cause of death through infectious disease.1 During the decade 1919–1928, 15,436 Australian children under one year of age died from gastroenteritis, accounting for almost 20% of deaths in this age group.2
Over time, the adoption of treatment using rehydration and intravenous drips reduced mortality rates, however measures such as antibiotics, improvements in water quality, sanitation and hygiene appeared to have limited effect. Consequently, infant diarrhoea was still a major problem by the 1950s with up to 25,000 cases occurring in Australia each year, a 1–2% mortality rate for infants generally, including those that were otherwise healthy, and up to 60% mortality for babies born prematurely.3
Clinicians and researchers had good evidence – such as the occurrence of seasonal epidemics4 – that the cause of acute diarrhoea was an infectious agent such as bacteria, a parasite or virus. However, there were also other potential non-infectious causes including food intolerances, premature birth and malnutrition. The complexity of this situation hampered investigations and attempts to control infection.5
Absence of a cause also created other problems for clinicians. In 1969, NHMRC’s Council was informed that general practitioners, in dealing with a case of acute diarrhoea in a young child, were under strong pressure to prescribe something and that their tendency was to prescribe antibiotics, despite this treatment’s lack of effect in most cases and negative effect on helpful intestinal bacteria.
By the early 1970s, researchers had reached an impasse, with one research team noting, after reviewing the literature, that, 'In spite of the diligent search for viral and/or bacterial pathogens, the majority of cases of gastroenteritis in infants less than two years of age remain unexplained'.
Recognising that finding a virus or bacteria in an infant’s stool would not demonstrate that it was the cause of the gastroenteritis unless all other possible pathogens had been excluded, they stated that, 'Unless potentially new pathogens are discovered or remarkably new techniques become available, additional studies like those described in this report for infectious agents will not be fruitful.'6
It was to be NHMRC-funded researchers in Australia who provided both.
Investment
Since its inception in 1926 as the Federal Health Council, NHMRC has invested time and resources, both directly and indirectly, towards solving the problem of infant diarrhoea.
The investment of time included periodic discussions at meetings of NHMRC’s Council and committees. For example, in 1950, Council – which includes the chief health and medical officers of each Australian state and territory – agreed to make infant diarrhoea a nationally notifiable disease.7
NHMRC also funded research into finding solutions, including through support for Sydney Rubbo at the University of Melbourne and the Gastroenterology Research Unit at the RCH led by Charlotte Anderson. Researchers whose work was significant for delivering impacts related to infant diarrhoea and who were funded by NHMRC included Ruth Bishop, Rudge Townley, Graeme Barnes, Ian Holmes, Barbara Coulson, Mike Dyall-Smith, Sue Rodger, Roger Schnagl, Carl Kirkwood and Julie Bines.
Ruth Bishop’s salary was supported by NHMRC-grants during the early stages of her career.

The PDF poster version of this case study includes a graphical timeline showing NHMRC grants provided and other events described in the case study.
Research
In 1968, when Ruth Bishop joined RCH’s Gastroenterology Research Unit as a Research Fellow and bacteriologist, the unit’s major research focus was on understanding chronic gastrointestinal illness such as coeliac disease and cystic fibrosis. Nonetheless, acute diarrhoea was a serious issue for the hospital: there was a special ward for gastroenteritis that was sometimes overflowing with patients. Bishop was therefore tasked with trying to find a cause.
Reviewing the enormous quantity of available literature,8 Bishop understood that the cause must be an infectious agent and, like many of her predecessors, she searched for causes of acute gastroenteritis within patient stool samples. Again, as with her predecessors, this search proved unsuccessful, but Bishop then looked for the cause in a new place.
RCH gastroenterologists Rudge Townley and Graeme Barnes together with staff from RCH’s Biomedical Engineering Department had developed a world-leading capability: they had found a way to safely take biopsies (small tissue samples) from an infant’s duodenal mucosa (the tissue of the upper intestine, just below the stomach). During the period 1968-1972, Townley and Barnes had undertaken almost 1,200 biopsies of this area in children, looking for causes of chronic diarrhoea.9
Prior to this innovation there had been few reports published on the state of the small intestine in acute gastroenteritis because it had been too difficult to obtain such biopsies safely.9 However, when Barnes and Townley used their new technique to answer this question they found evidence of severe cellular and tissue damage, but no known bacterial or viral pathogens.10
Bishop recognised that these biopsies might contain the infectious agent and that if it wasn’t bacterial then it was most likely a virus. So, she discussed this idea with Ian Holmes, within the University of Melbourne’s Department of Microbiology, who was by this time a world-leading expert at searching for viruses.
Bishop asked Geoff Davidson, a Gastroenterology Research Fellow at RCH, to perform more duodenal biopsies and Davidson then sent the specimens to Holmes for analysis.
Using cutting-edge electron microscopy, Holmes and colleagues, Brian Ruck and Roger Schnagl, examined the duodenal biopsies and visually identified the presence of a distinct new member of an established virus family, which came to be called a rotavirus, from the Latin word for wheel. The Holmes group rapidly confirmed this identification through characterisation of the rotavirus genes and proteins, using their newly-developed techniques.11
Bishop’s team announced the discovery in 1973 and a year later they published a method for locating rotavirus particles in patient stool samples, making it possible for laboratories around the world to identify the virus without taking duodenal biopsies.12,13
By 1975, after a year-long survey undertaken by Davidson of children admitted to RCH with acute enteritis, the new virus was found via electron microscopy in stool samples of over 50% of patients. Following their initial announcement, identical viruses associated with acute gastroenteritis had also been identified across Australia and in numerous countries worldwide. This provided strong evidence that rotaviruses were the most important world-wide cause of acute gastroenteritis in young children.14
As subsequent research was to demonstrate, rotaviruses are highly infectious: an infected person can shed more than 10 billion viral particles per ml of faeces. Transmission of rotavirus infection in a susceptible community can occur extremely rapidly through the air, water and contact with surfaces. Moreover, rotaviruses are very durable, being able to survive for weeks outside of the body.15 These features of the virus revealed not only why it was so difficult to control, but also that development of a vaccine was the only likely way of doing so.
Translation
From 1975, the University of Melbourne team made key contributions to the characterisation of the genome and proteins of human and animal rotaviruses and discovered novel rotaviruses. They developed a range of new methods for virus isolation, virus detection in stools and immune responses to rotavirus that have now been used for decades at RCH and elsewhere.
Sequential patterns of appearance of human rotavirus strains were discovered. Together, the RCH and University of Melbourne teams identified a distinct, non-disease-causing strain (RV3) circulating amongst newborn babies housed in the Royal Women’s Hospital, Melbourne.16 This led to a new breakthrough by the RCH team, who had undertaken a longitudinal study of these newborn babies. In 1983, they announced that infants infected with rotavirus as newborns were protected from severe symptoms of diarrhoea on reinfection.17 This finding provided strong evidence that a live, attenuated rotavirus vaccine would be effective.
Further evidence was provided by Barbara Coulson and colleagues at RCH, who demonstrated the importance of pre-existing gut immune responses to rotavirus for reducing rotavirus reinfection in older children.18 Coulson led the production in quantity of a large range of monoclonal antibodies to human rotaviruses that can distinguish the degree of relatedness between rotaviruses, leading to identification of the virus protein regions critical for eliciting protection, and novel assays for rotavirus relatedness using stool samples.19 These antibodies have been used globally for vaccine development and trials.
For several decades, these antibody assays have also been crucial for the Australian Rotavirus Surveillance Program monitoring, conducted by Carl Kirkwood. The distinct nature of the RV3 rotavirus strain was directly shown by Coulson using RV3-specific monoclonal antibodies, facilitating RV3 patenting for vaccine development.
In 2006, two oral rotavirus vaccines were licensed for use in Australia – Rotarix® and RotaTeq®. The Northern Territory introduced a funded rotavirus vaccine program in October 2006 and from July 2007 both vaccines were incorporated into Australia’s National Immunisation Program (NIP) for all Australian infants. Initially, each of the eight Australian states and territories implemented one or other of the two vaccines; from July 2017 all jurisdictions only administered Rotarix under the NIP.20
The discovery of the unusual asymptomatic newborn strain in 1983 also provided the basis for the development of the RV3-BB rotavirus vaccine based on this strain. Led by Julie Bines and the team from MCRI, the RV3BB vaccine has been studied in clinical trials in Australia, New Zealand, Indonesia and Malawi with the aim to target protection for severe rotavirus disease in babies from birth.21, 22 Indonesian manufactured RV3-BB vaccine is planned for introduction into the Indonesian National Immunisation Program in 2024.
Outcomes and impacts
The rotavirus discovery and vaccine development are among the most important contributions made by medical research to global human health.
In Australia, before the oral rotavirus vaccine program, rotavirus annually caused more than 10,000 hospitalisations, 22,000 emergency department (ED) presentations and 115,000 visits to general practitioners for children less than 5 years old, with an estimated annual cost of A$30 million.21
In the first three years following vaccine uptake, there was a greater than 64% decrease in rotavirus-related hospitalisations among vaccine eligible children, and 40 to 60% decrease in rotavirus-related hospitalisations and ED presentations among children too old to have been vaccinated (typically children greater than 2 years old).21
The rotavirus vaccine was so effective at reducing hospitalisations that gastroenteritis wards were able to close.23,24,25,26
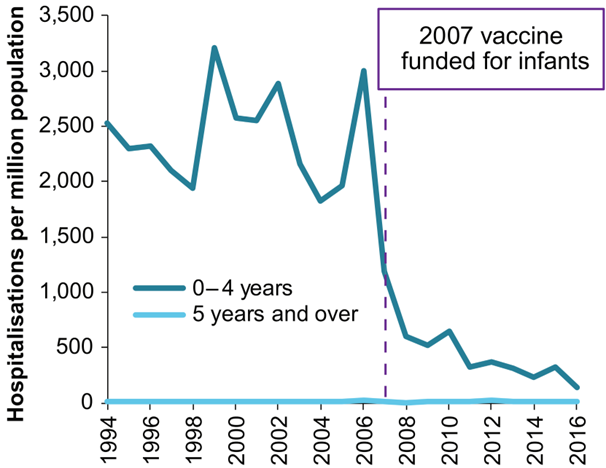
The rotavirus vaccine provided a range of additional benefits including reduced workload for clinicians and hospital staff, and their consequent increased capacity to provide other types of healthcare. Also achieved were total healthcare cost savings in Australia of about A$65 million in excess of the total cost of the vaccination program between 2007 and 2012.22
Internationally, rotavirus vaccine use in national immunisation programmes has led to a significant and sustained reduction in the proportion of hospital admissions for acute gastroenteritis due to rotavirus among children younger than 5 years.28 By 2024, over 115 countries had introduced rotavirus vaccines into their national routine immunisation programs. The proportion of hospital admissions for diarrhoea due to rotavirus among children less than 5 years of age decreased from approximately 40% in the pre-vaccine era to 20-25% following the introduction of vaccines.29
Researchers
Professor Ruth Bishop AC
Ruth Frances Bishop (1933-2022) graduated from the University of Melbourne in 1961 with a PhD in microbiology. She was a Research Fellow in the Department of Surgery, University of Liverpool UK (1962-1965) and then, after returning to Australia, became a Research Assistant then Research Fellow in the RCH Department of Gastroenterology (1968-1999). Following this, Bishop was appointed a Senior Principal Research Fellow at the RCH Research Foundation and in 1995 was made a Professorial Fellow in the University of Melbourne’s Department of Paediatrics.
Bishop worked for the World Health Organisation (WHO) including as: Chair of the Steering Committee on Viral Diarrhoeal Diseases; Chair of a Scientific Working Group on Immunology, Microbiology and Vaccine Development; Director of the WHO Regional Collaborating Rotavirus Laboratory; and as Special Adviser to the WHO Vaccine Development Program. Bishop was also a member of the Rotavirus Working Group for the Bill and Melinda Gates Foundation’s Children’s Vaccine Program. In 2021, Bill Gates published a statement that Bishop’s work was a catalyst for the establishment of the Foundation.
Bishop was made an Officer (1996) and then a Companion (2019) of the Order of Australia, received the Clunies Ross National Science and Technology Award (1998), the Children’s Vaccine Initiative Award (WHO Geneva 1998) and the Florey Medal (2013), among other awards.
Professor Graeme Barnes AO
After receiving medical training at the University of Otago, New Zealand, Barnes commenced training in paediatrics at RCH in 1970. He later received an RCH Scholarship and, in 1973, a Heinz Travelling Fellowship to travel to the UK. In 1975, Barnes became Director of Gastroenterology at RCH. In 1995, Barnes became Scientific Director of the RCH Research Foundation. A review of the Foundation led by Barnes culminated in amalgamation with the Murdoch Institute to form MCRI.
In 2020, Barnes was appointed an Officer of the Order of Australia. Other awards include The Vernon Collins Oration Medals (2008 and 2011) and The Howard Williams Oration Medal (2008).
Associate Professor Ian Holmes
Ian Hamilton Holmes (1935-2010) completed an undergraduate science degree at the University of Melbourne and then, in 1961, a PhD at the Australian National University. Holmes was a senior member of the University of Melbourne’s academic staff from 1963-2000, but also worked overseas in Venezula and Glasgow during this period.
From 1973 until the 1990s he took part in several groups within the International Committee for the Taxonomy of Viruses, including chairing the Reoviridae study group from 1987 to 1993.
Holmes received the University of Melbourne’s David Syme Research Prize (1977) and shared (with Ruth Bishop) the Clunies Ross National Science and Technology Award (1998).
Professor Geoff Davidson PSM
Geoffrey Paul Davidson (1942-2020) graduated from the University of Adelaide medical school in 1968, winning the paediatrics prize, and went on to train at children’s hospitals in Adelaide, Melbourne and Toronto. Davidson commenced at RCH as a Gastroenterology Research Fellow in 1973 and then, returning to Adelaide in 1978, was appointed founding Director of Paediatric and Adolescent Health at the Women’s and Children’s Hospital. Davidson was awarded the Public Service Medal in 1997.
Associate Professor Barbara Coulson
Barbara Sue Coulson graduated from the University of Melbourne with a Bachelor of Science and then, in 1982, a PhD in Microbiology on rotavirus infection in infants. Coulson’s PhD research was supervised by Ian Holmes and she then undertook early postdoctoral research in collaboration with Ruth Bishop. Coulson was Senior Research Officer (1983-1990) and then Research Fellow (1991-1995) in the RCH Department of Gastroenterology. In 1996, Coulson relocated her group to the University of Melbourne where in 2009 she was appointed Associate Professor and Reader. Since 2019, Coulson has been Honorary Principal Research Fellow at the University. Coulson is a member of the Editorial Board of the Journal of Virology.
Coulson’s research group discovered several receptors mediating rotavirus entry into cells and – in collaboration with Professor Mark von Itzstein and colleagues at The Institute for Glycomics, Griffith University – worked on rotavirus drug development, leading to the world-first molecular characterisation of the binding of human rotaviruses to several novel carbohydrate receptors. Coulson was integral to the University of Melbourne team that in 2000 discovered a link between childhood rotavirus infection and accelerated type 1 diabetes development. The existence of this link was confirmed in several countries, including Australia, when rotavirus vaccination was later shown to be associated with reduced childhood type 1 diabetes development.
Professor Julie Bines
Julie Bines graduated in medicine (MBBS) from Monash University, becoming a Fellow of the Royal College of Physicians in 1990. Bines was a Clinical and Research Fellow in the Combined Program of Gastroenterology and Nutrition at Boston Children’s Hospital and Massachusetts General Hospital in Boston and Post-doctoral Fellow at Massachusetts Institute of Technology (1988-1991). Bines was awarded the degree of Doctor of Medicine at the University of Melbourne in 2000, was appointed the Victor and Loti Smorgon Professor of Paediatrics at the University of Melbourne in 2006 and is currently a Paediatric Gastroenterologist and Clinical Nutrition Consultant at RCH and Group Leader for the Enteric Diseases group at MCRI.
Bines led the development of the RV3-BB vaccine, including clinical trials in Australia, New Zealand, Indonesia and Malawi. Bines is Director of the WHO Collaborative Centre for Child Health and the WHO Rotavirus Regional Reference Laboratory for the Western Pacific Region and is a member of the WHO Expert Advisory Committee for Vaccines to address Anti-Microbial Resistance. Bines was the recipient of the 2022 Australian Museum Eureka Prize for the development of the RV3-BB vaccine.
Other researchers
Additional researchers who contributed to the impacts described in this case study include Donald Cameron OAM, Associate Professor Mike Dyall-Smith, Associate Professor Carl Kirkwood, Dr Sue Rodger and Dr Roger Schnagl.
Partners
This case study was developed with input from Professor Julie Bines, Associate Professor Barbara Coulson and Professor Graeme Barnes, in partnership with Murdoch Children’s Research Institute, and in consultation with the University of Melbourne and The Royal Children’s Hospital, Melbourne.



References
The information and images from which impact case studies are produced may be obtained from a number of sources including the case study partner, NHMRC’s internal records and publicly available materials. Key sources of information consulted for this case study include:
1Australian Institute of Health and Welfare. Mortality over the twentieth century in Australia: Trends and patterns in major causes of death. Mortality Surveillance Series no. 4. AIHW cat. no. PHE73. Canberra: AIHW. 2005
2Campbell, JM. Report on maternal and child welfare in Australia. Department of Health. 1929. In Federal Health Council, Report of 4th meeting. Canberra: Australian Government. 1930
3Rubbo SD. Epidemiology of Infectious Diarrhoea. Medical Journal of Australia. 1952;1(13):425-8
4Ferris AA. The epidemiology of infective diarrhoea. Australian Journal of Science. 1965; 28: 187–92
5National Health and Medical Research Council. Report of 69th meeting. Volume 2. Australia. 1969.
6Cramblett HG, Azimi P, Haynes RE. The etiology of infectious diarrhea in infancy with special reference to Enteropathogenic E. coli. Annals of the New York Academy of Sciences. 1971; 176: 80–92
7National Health and Medical Research Council. Report of 30th meeting. Australia, 1950 p42
8Mushin R. The Bacteriology of Infectious Diarrhoea. Medical Journal of Australia. 1952;1(13):428-32
9Townley RRW, Barnes GL. Intestinal biopsy in childhood. Archives of Disease in Childhood. 1973; 48: 480–2.
10Barnes GL, Townley RRW. Duodenal mucosal damage in 31 infants with gastroenteritis. Archives of Disease in Childhood. 1973; 48: 343–9
11Rodger SM, Schnagl RD, Holmes IH. Biochemical and biophysical characteristics of diarrhea viruses of human and calf origin. Journal of Virology. 1975 Nov;16(5):1229-35
12Bishop R, Davidson GP, Holmes IH, Ruck BJ. Detection of a new virus by electron microscopy of faecal extracts from children with acute gastroenteritis. The Lancet. 1974 Feb 2;303(7849):149-51
13Bishop R. Ruth Bishop: rotaviruses and vaccines. Interview by Amanda Tattam. Lancet. 1999 May 29;353(9167):1860
14Davidson GP, Bishop RF, Townley RR, Holmes IH, Ruck BJ. Importance of a new virus in acute sporadic enteritis in children. The Lancet. 1975 Feb 1;305(7901):242-6
15Bishop RF. Natural history of human rotavirus infection. Viral Gastroenteritis. 1996 Jan 1:119-28
16Rodger SM, Bishop RF, Birch CH, McLean BA, Holmes IH. Molecular epidemiology of human rotaviruses in Melbourne, Australia, from 1973 to 1979, as determined by electrophoresis of genome ribonucleic acid. Journal of Clinical Microbiology. 1981 Feb;13(2):272-8
17Bishop RF, Barnes GL, Cipriani E, Lund JS. Clinical immunity after neonatal rotavirus infection: a prospective longitudinal study in young children. New England Journal of Medicine. 1983 Jul 14;309(2):72-6
18Coulson BS, Grimwood K, Hudson IL, Masendycz PJ, Lund JS, Bishop RF and Barnes GL (1992). Role of coproantibody in clinical protection of children during reinfection with rotavirus. Journal of Clinical Microbiology. 30: 1678-1684
19Coulson BS, Unicomb LE, Pitson GA and Bishop RF (1987). Simple and specific enzyme immunoassay using monoclonal antibodies for serotyping human rotavirus. Journal of Clinical Microbiology. 25: 509-515
20Middleton BF, Danchin M, Fathima P, Bines JE, Macartney K, Snelling TL. Review of the health impact of the oral rotavirus vaccine program in children under 5 years in Australia: 2006 - 2021. Vaccine. 2023 Jan 16;41(3):636-648
21Bines JE, At Thobari J, Satria CD, Handley A, Watts E, Cowley D, Nirwati H, Ackland J, Standish J, Justice F, Byars G, Lee KJ, Barnes GL, Bachtiar NS, Viska Icanervilia A, Boniface K, Bogdanovic-Sakran N, Pavlic D, Bishop RF, Kirkwood CD, Buttery JP, Soenarto Y. Human Neonatal Rotavirus Vaccine (RV3-BB) to Target Rotavirus from Birth. New England Journal of Medicine. 2018 Feb 22;378(8):719-730
22Bines JE, Danchin M, Jackson P, Handley A, Watts E, Lee KJ, West A, Cowley D, Chen MY, Barnes GL, Justice F, Buttery JP, Carlin JB, Bishop RF, Taylor B, Kirkwood CD; RV3 Rotavirus Vaccine Program. Safety and immunogenicity of RV3-BB human neonatal rotavirus vaccine administered at birth or in infancy: a randomised, double-blind, placebo-controlled trial. Lancet Infectious Diseases. 2015 Dec;15(12):1389-97
23Fathima P, Jones MA, Moore HC, Blyth CC, Gibbs RA, Snelling TL. Impact of Rotavirus Vaccines on Gastroenteritis Hospitalizations in Western Australia: A Time-series Analysis. J Epidemiol. 2021 Aug 5;31(8):480-486
24Heinsbroek E, Hungerford D, Cooke RPD, Chowdhury M, Cargill JS, Bar-Zeev N, French N, Theodorou E, Standaert B, Cunliffe NA. Do hospital pressures change following rotavirus vaccine introduction? A retrospective database analysis in a large paediatric hospital in the UK. BMJ Open. 2019 May 15;9(5)
25Hartwig, S., Uhari, M., Renko, M. et al. Hospital bed occupancy for rotavirus and all cause acute gastroenteritis in two Finnish hospitals before and after the implementation of the national rotavirus vaccination program with RotaTeq®. BMC Health Serv Res 14, 632 (2014)
26Refer https://www.rch.org.au/alumni/alumni_profiles/Bishop,_Ruth_AC/. Accessed 13 February 2024
27Australian Institute of Health and Welfare. Rotavirus in Australia. Vaccine-preventable diseases fact sheets. 2018. Cat no. PHE 236
28Aliabadi N, Antoni S, Mwenda JM, Weldegebriel G, Biey JN, Cheikh D, Fahmy K, Teleb N, Ashmony HA, Ahmed H, Daniels DS. Global impact of rotavirus vaccine introduction on rotavirus hospitalisations among children under 5 years of age, 2008–16: findings from the Global Rotavirus Surveillance Network. The Lancet Global Health. 2019 Jul 1;7(7):e893-903
29Glass RI, Tate JE, Jiang B, Parashar U. The rotavirus vaccine story: from discovery to the eventual control of rotavirus disease. The Journal of Infectious Diseases. 2021 Oct 1;224(Supplement_4): S331-42.