Chikungunya is a mosquito-transmitted viral disease characterised by sporadic, unpredictable outbreaks. Due to international travel and the spread of potential disease-carrying vectors such as mosquitos, chikungunya virus (CHIKV) infections have been identified in over 125 countries.1 Over the past 20 years, more than 10 million chikungunya virus infections have been reported, highlighting CHIKV as a significant global health threat. An international consortium involving NHMRC-funded researchers at Griffith University has developed 2 vaccines against CHIKV.
Origin
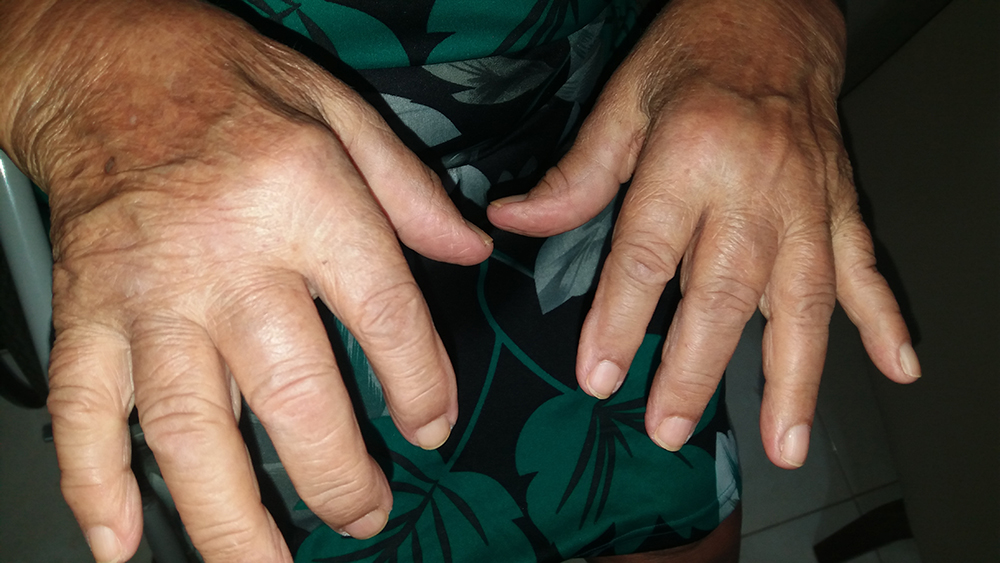
CHIKV is a mosquito-borne virus that typically causes symptoms 3–7 days after a bite from an infected mosquito. The most common symptoms include fever and joint pain, with some patients also experiencing headache, muscle pain, joint swelling, or rash. While some people recover within a week, persistent disease may occur in more than half of patients, causing debilitating severe joint pain for months to years following acute illness. This not only significantly affects patient health and quality of life but can also severely strain local economies and healthcare systems during outbreaks in populations whose immune systems have not previously been exposed to the virus.
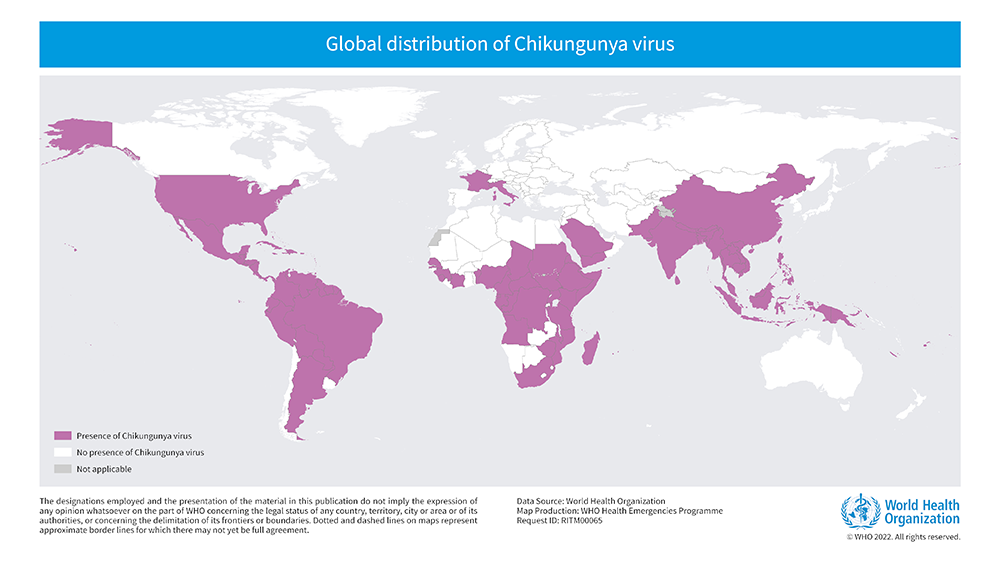
First reported in Tanzania in 1952, CHIKV spread through the Indian Ocean islands, India, Southeast Asia and Asia-Pacific nations close to Australia, including Papua New Guinea. In 2013, CHIKV was detected in Central and South America and has continued to circulate in the region since then. While regions such as the European Union (EU) and the United States of America (USA) have reported few locally transmitted outbreaks, they have experienced many imported cases. More than a billion people live in chikungunya-endemic areas and climate change could expand the regions suitable for mosquito vectors, increasing the at-risk population.3
Severe disease and death caused by CHIKV are most common in babies, adults with underlying conditions such as high blood pressure, diabetes, or heart disease, and older people. Although death from CHIKV was previously considered rare, the case-fatality ratio has now been found to be up to 1 per 1000.4
A significant outbreak in La Réunion during 2005-2006 affected over 250,000 people, about one-third of the population, and resulted in 250 fatal cases. More than 4,500 excess deaths were reported in India and Mauritius during their 2005–2006 CHIKV epidemics, with the national burden of CHIKV disease in India estimated to be 25,588 disability-adjusted life years lost during this epidemic. In total, 14,000 CHIKV-related deaths were estimated between 2014 and 2016 during epidemics in Brazil, the Dominican Republic, Puerto Rico, Guadeloupe and Martinique.5,6,7
The numerous outbreaks and imported cases, which highlight the virus’s potential to spread globally, prompted the creation of the EU-led Integration of Chikungunya Research (ICRES) consortium.
Investment
Australia’s involvement in ICRES has been led by Suresh Mahalingam and Adam Taylor at Griffith University Institute for Biomedicine and Glycomics, and Brett Lidbury at the University of Canberra, who have received a variety of NHMRC grants to support their research, including fellowships, Project Grants and an NHMRC-EU Collaborative Grant. Joseph Fraser, at Royal Melbourne Hospital and the University of Melbourne, received long-term funding from NHMRC to support research related to polyarthritis caused by Ross River Virus (RRV). Lynn Dalgarno at the Australian National University (ANU) received NHMRC funding to investigate RRV.
The Griffith University team’s research was also supported by funding from the Australian Research Council, Department of Industry, Science and Resources (Australia-India Strategic Research Fund), and the EU Framework Programme - Health and the National Foundation for Medical Research and Innovation.
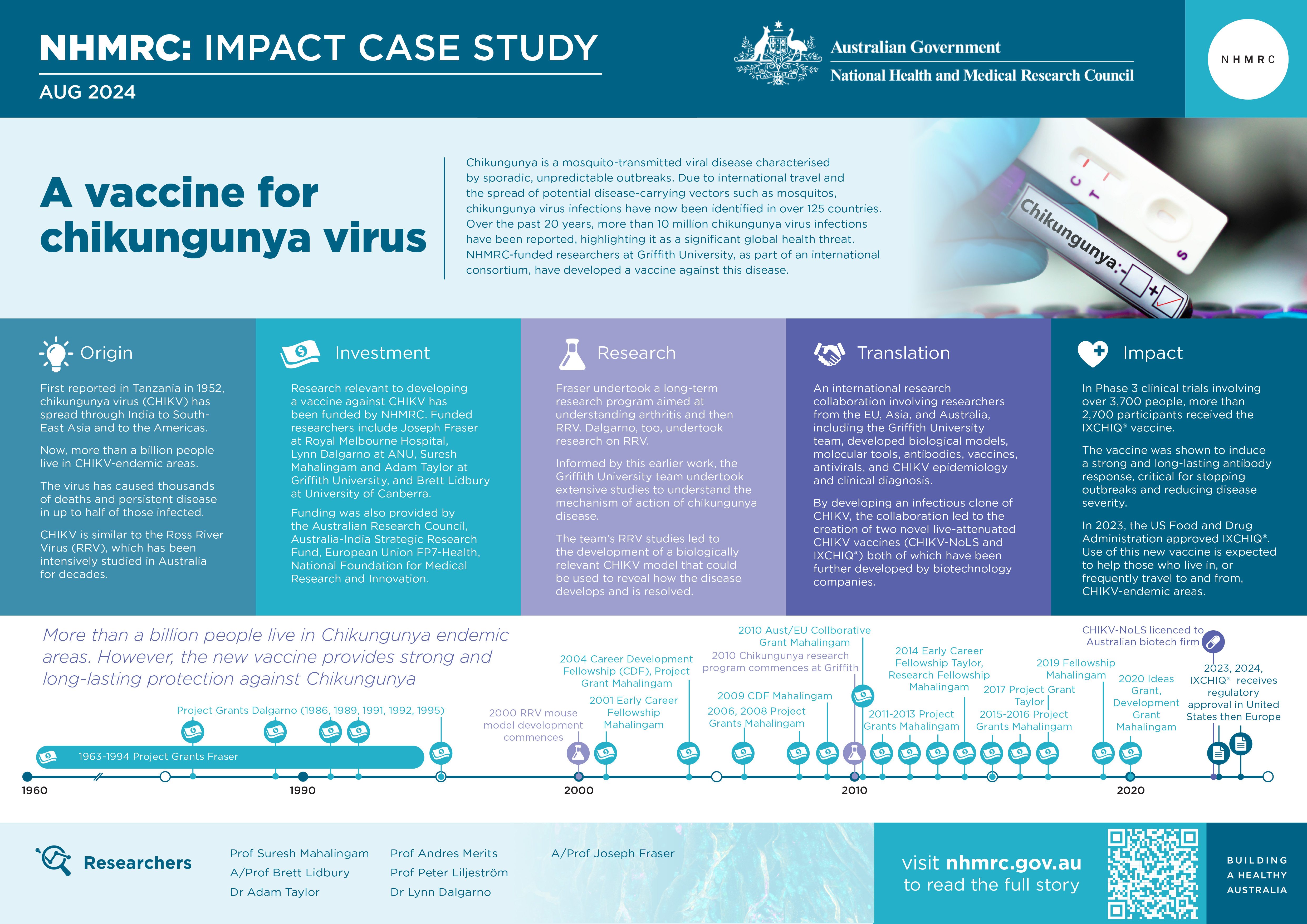
The PDF poster version of this case study includes a graphical time showing NHMRC grants provided and other events described in the case study.
Research
Commencing in the 1970s, Dr Joseph Fraser, an infectious disease and rheumatology clinician at Royal Melbourne Hospital and the University of Melbourne, embarked on a long-term investigation into the causes of polyarthritis in his patients, making several key insights into RRV disease.
Around the same time, Professors Cedric Mims and Ian Marshall at ANU’s John Curtin School of Medical Research (JCSMR) conducted early research aiming to understand how RRV infection leads to disease. However, their work was hampered by the lack of an animal model that effectively mimicked human disease.
In 1995, Brett Lidbury commenced research on RRV as a postdoctoral fellow with JCSMR researcher Lynn Dalgarno, a virologist renowned for his contributions to alphavirus research, particularly RRV.
Lidbury and Dalgarno conducted studies on RRV disease formation and confirmed an earlier finding (made by Marshall and colleague Andrew Hapel) that reducing a type of immune cell called macrophages reduced the symptoms of RRV disease. This provided the first evidence that RRV disease was driven by a malfunctioning immune system, a finding further confirmed when Mahalingam and Lidbury identified factors that regulate other immune cells called monocytes that contribute to RRV disease. Further collaborations with Fraser were crucial in establishing the role of the immune system in this disease. In 2000, Mahalingam and Lidbury’s research led to the establishment of a mouse model for RRV-induced disease and the understanding of key disease pathways, development and resolution.
RRV is closely related to CHIKV, causing similar symptoms and outbreaks in Australia and the Asia-Pacific region. The mouse model of RRV disease developed by Mahalingam, Lidbury and colleague Mark Heise enabled studies exploring CHIKV infection and disease progression. In 2010, Mahalingam moved to Griffith University and established a CHIKV research program which included establishing a CHIKV mouse model based on the team’s learnings from their RRV studies.
The ICRES consortium, involving researchers from the EU, Asia, and Australia, including the Mahalingam research team, supported research into the development of animal models, molecular tools, antibodies, vaccines, antivirals, and CHIKV epidemiology and clinical diagnosis. A key component of the consortium’s work was the development of an infectious clone of CHIKV, forming the basis for the creation of two novel live-attenuated CHIKV vaccines: CHIKV-NoLS by Taylor and Mahalingam at Griffith University, and CHIKV-5nsP3 by Peter Liljeström from the Karolinska Institute and Andres Merits from the University of Tartu.
Translation
CHIKV-NoLS has demonstrated safety in numerous pre-clinical studies and elicits a strong protective immune response in animal models. A patent application covering the vaccine candidate was filed in eight countries and has so far been granted in Australia, USA, Indonesia, China, the Philippines and India, with applications still pending in Brazil and Thailand. In December 2023, the intellectual property for the vaccine was licensed to an Australian biotech company focused on developing vaccines for emerging viruses. CHIKV-NoLS is currently progressing to the commencement of clinical trials to support its further development.
CHIKV-5nsP3 has shown safety in various pre-clinical studies, generating a protective immune response in animal models. The vaccine candidate, subsequently known as VLA1553, was further developed by Valneva, a French biotech company specialising in developing and commercialising vaccines for infectious diseases to address unmet medical needs. To make VLA1553 more accessible in low- and middle-income countries, Valneva and Instituto Butantan in Brazil signed an agreement in January 2021 for the development, manufacturing and marketing of VLA1553. In 2019, Valneva was supported by a European program, the Coalition for Epidemic Preparedness Innovations, to carry out Phase 3 clinical trials. VLA1553 demonstrated a robust immune response sustained for 24 months by 97% of participants and was equally durable in younger and older adults.
Outcomes and impacts
In November 2023, VLA1553 received accelerated approval from the US Food and Drug Administration under the brand name IXCHIQ®. In May 2024, the vaccine also received a positive opinion from the European Medicines Agency. As of August 2024, IXCHIQ® is the only marketed chikungunya vaccine available for preventing CHIKV disease in individuals 18 years of age and older who are at increased risk of exposure.
In Phase 3 clinical trials involving over 3,700 people, more than 2,700 participants received the vaccine, while about 1,000 received placebo. The results showed that the vaccine induces a strong and long-lasting antibody response, critical for stopping outbreaks and reducing disease severity. Among those vaccinated, the antibody response was very high, at about 96 to 98 percent, directly benefiting travellers and people living in endemic areas worldwide.
While conducting an efficacy trial for the vaccine is challenging due to the unpredictable and rapid nature of chikungunya outbreaks, the vaccine is expected to significantly improve people's lives.
The vaccine is particularly important to Australians because of Australia’s geographic proximity to CHIKV-endemic countries in Southeast Asia and the Pacific Islands, where outbreaks are frequent. This proximity increases the risk of the virus being introduced into Australia through travel and trade. Australians frequently travel to and from these CHIKV-endemic areas, and infected travellers could easily bring the virus back, leading to potential local transmission.
The primary mosquito vector for CHIKV, Aedes aegypti, is present in some parts of Australia, particularly in Queensland, while another vector – Aedes albopictus – is found mainly in the northern regions such as the Torres Strait. These mosquitoes could readily transmit the virus if they bite an infected individual. The tropical and subtropical climates of Australia, where Aedes mosquitoes thrive, provide suitable environments for the virus to spread if introduced. Consequently, research on CHIKV disease, vaccines and treatments have enhanced Australia’s capacity to respond to potential future outbreaks.
Researchers
Professor Suresh Mahalingam
Suresh Mahalingam received a PhD from the JCSMR in 1999. In 2003, he commenced research at the University of Wollongong. In 2005, he became an Associate Professor at the University of Canberra. Mahalingam is currently a professor of viral immunology at Griffith University where he leads the Emerging Viruses, Inflammation and Therapeutics Group and is a Director of the Global Virus Network Centre of Excellence in Arbovirus Research. He is a Fellow of the American Academy of Microbiology, the Royal College of Pathologists of Australasia, and serves as the American Society for Microbiology Country Ambassador to Australia.
Associate Professor Brett Lidbury
Brett Lidbury received a PhD from the JCSMR in 1993. This was followed by a period working on a commercially funded project at the Australian Institute of Mucosal Immunology, NSW. He studied alphavirus pathogenesis during postdoctoral studies in 1995 with Lynn Dalgarno at ANU. In October 1996, Lidbury commenced a lectureship in the School of Human and Biomedical Sciences, University of Canberra. He is presently an Associate Professor at the National Centre for Epidemiology and Population Health, College of Health and Medicine at ANU. Lidbury was a nominee for 2016 ACT Scientist of the Year and is a Fellow of the Faculty of Science, The Royal College of Pathologists of Australasia.
Dr Adam Taylor
Adam Taylor graduated with a degree in Human Genetics from Newcastle University, UK, in 2007 and then, in 2011, with a PhD in Molecular and Cellular Biology from the University of Leeds. Between 2011 and 2023, Taylor led teams investigating immune mediators of viral disease and vaccine development at Griffith University. In 2021, he was awarded an Australian Institute of Policy and Science Queensland Young Tall Poppy Science Award.
Professor Andres Merits
Andres Merits received his PhD in 1994 at the University of Tartu, Estonia, then undertook postdoctoral research at the University of Helsinki, Finland and became a group leader and professor in 2003. He received the State Award (Estonia) in Chemistry and Molecular Biology (2015) and was elected a Research Professor of the Estonian Academy of Sciences. Between 2020-2021, he was a member of the Advisory Board of the Estonian Government on COVID-19 and established SARS-CoV-2 research in Estonia.
Professor Peter Liljeström
Peter Liljeström is a professor at the Karolinska Institute in Sweden. He received his PhD in 1986 at the University of Helsinki, Finland. He was appointed assistant professor in molecular biology in 1987 and moved to the Karolinska Institute in 1987, becoming associate professor in 1993 and full professor in 1996. As a joint position, he was also appointed Chief of the Department of Vaccines at the Swedish Institute for Infectious Disease Control (SMI) in 1996. Having restructured Sweden's vaccination program, he retired from the position in 2012.
Dr Lynn Dalgarno
Lynn Dalgarno graduated from the University of Melbourne in 1958, with a Bachelor of Science in Agriculture, and then, in 1962, a PhD from ANU. Between 1963 and 1967, Dalgarno was a postdoctoral researcher in London, Paris, and the California Institute of Technology. In 1968 Dalgarno became a Senior Lecturer at ANU, and was then a Reader from 1983 until 1996, when he subsequently became a Research Fellow.
Associate Professor Joseph Fraser
Joseph Robert (Bob) E Fraser (d.2013) was a rheumatologist and clinician researcher with an active research program at the Department of Medicine, Royal Melbourne Hospital, University of Melbourne in the 1970s and 1980s. He also worked at regional hospitals in the Murray-Goulburn Valley, Victoria.
Partner
This case study was developed with input from Professor Suresh Mahalingam and in partnership with Griffith University.

References
The information and images from which impact case studies are produced may be obtained from a number of sources including our case study partner, NHMRC’s internal records and publicly available materials. Key sources of information consulted for this case study include:
1 de Souza WM, de Lima ST, Mello LM, Candido DS, Buss L, Whittaker C, Claro IM, Chandradeva N, Granja F, de Jesus R, Lemos PS. Spatiotemporal dynamics and recurrence of chikungunya virus in Brazil: an epidemiological study. The Lancet Microbe. 2023 May 1;4(5):e319-29
2 Ng WH, Amaral K, Javelle E, Mahalingam S. Chronic chikungunya disease (CCD): clinical insights, immunopathogenesis and therapeutic perspectives. QJM: An International Journal of Medicine. 2024 Feb 20:hcae028.
3 Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. Chikungunya: a re-emerging virus. Lancet. 2012 Feb 18;379(9816):662-71
4 Mavalankar D, Shastri P, Bandyopadhyay T, Parmar J, Ramani KV. Increased mortality rate associated with chikungunya epidemic, Ahmedabad, India. Emerging infectious diseases. 2008;14(3):412–5
5 Beesoon S, Funkhouser E, Kotea N, Spielman A, Robich RM. Chikungunya fever, Mauritius, 2006. Emerging infectious diseases. 2008;14(2):337–8.
6 Freitas ARR, Alarcon-Elbal PM, Donalisio MR. Excess mortality in Guadeloupe and Martinique, islands of the French West Indies, during the chikungunya epidemic of 2014. Epidemiology and infection. 2018;146(16):2059–65.
7 Burt FJ, Chen W, Miner JJ, Lenschow DJ, Merits A, Schnettler E, Kohl A, Rudd PA, Taylor A, Herrero LJ, Zaid A, Ng LFP, Mahalingam S. Chikungunya virus: an update on the biology and pathogenesis of this emerging pathogen. Lancet Infect Dis. 2017 Apr;17(4):e107-e117.