Colorectal adenocarcinoma, also known as colorectal cancer (CRC) or bowel cancer, is the second most common cause of cancer-related death in Australia. With one of the highest rates of CRC in the world, Australia was also the first country in the world to implement and sustain a national population-based organised CRC screening program using faecal immunochemical tests. NHMRC-funded researchers made key contributions to the program’s initial development and ongoing conduct.
Origin
CRC encompasses adenocarcinoma occurring in the colon and rectum. The process of developing CRC usually occurs in stages (Figure 1). During the first stage, the normal cells of the bowel multiply and develop into a pre-malignant lesion. The main premalignant lesions are adenomas or serrated lesions that often take the shape of a polyp. A small proportion of premalignant lesions may progress over a period (that may be as long as ten years) before becoming locally invasive – the key characteristic of a cancer. If left untreated, the cancer may then become more invasive and spread throughout the body.
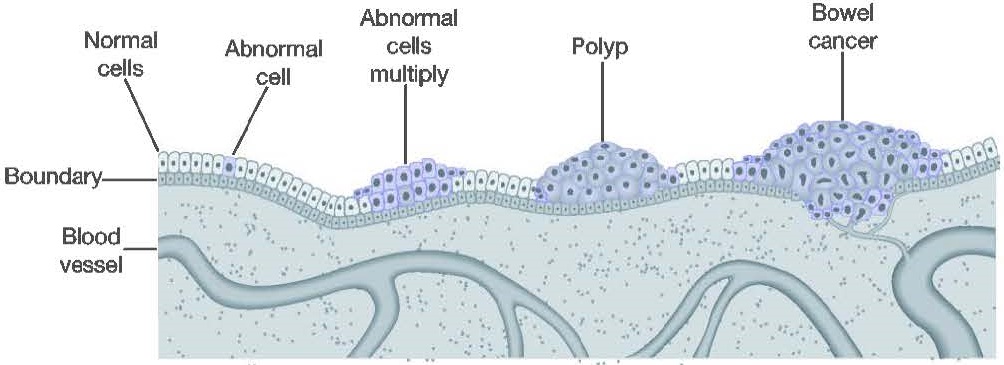
If a premalignant lesion is identified before it has become a cancer then it may be removed, in most cases during a colonoscopy, and so prevent possible progression to a cancer. This procedure is generally simple and safe. Cancer progresses through four stages (I-IV) characterised by increasing spread. However, most people harbouring a premalignant lesion or stage I or II CRC do not have symptoms that alert them to the problem.2
In order for death rates from CRC to decrease significantly, it is necessary to detect and remove premalignant lesions or CRC when in the curable stages (especially stages I and II where 5-year survival is better than 75%). Most people do not have symptoms at these stages, so detection and improved survival requires implementation of population-level screening. But to do this, a host of obstacles need to be overcome.2
Investment
By 1936, NHMRC had identified the importance of early cancer detection and treatment.3 In 1968, NHMRC agreed that cancer detection programs should focus on individuals at risk of particular cancers, and it also recognised CRC as one of the commonest causes of death from cancer in the community.4 By 1971, a major breakthrough occurred when a simple chemical test (called a guaiac-based faecal occult blood test or gFOBT) was developed that was able to detect non-visible traces of blood in stool: blood that could be a result of bleeding premalignant lesions and cancer.5
From this time onward, NHMRC provided a succession of grants related to CRC screening, including to researchers James St John, Graeme Young, Finlay Macrae and Glenn Salkeld, whose work would underpin development of what became the National Bowel Cancer Screening Program (NBCSP). Other sources of funding for their work included: Cancer Australia, State Cancer Councils, US National Institutes of Health, Australia Cancer Research fund, South Australian Strategic Health Research Program, Smith-Kline Diagnostics, Australian Gastrointestinal Trials Group, US National Cancer Institute, Flinders Foundation, Hospital Research Foundation, CSIRO, Eiken Chemical Company, Fujirebio and Enterix Australia.

The PDF poster version of this case study includes a graphical time showing NHMRC grants and other events described in the case study.
Research
During the early 1980s, while working within the Department of Gastroenterology at The Royal Melbourne Hospital, and the Department of Medicine at the University of Melbourne, St John, Young and Macrae began developing a basic and clinical research network with the aim of improving understanding of the nature of bleeding from colorectal neoplasms (especially early stage cancers), the chemical fate of haemoglobin (a protein in red blood cells) in the gut, and how emerging technologies for detection of faecal occult blood might improve screening test accuracy.
During the following decades, that network expanded to include many national and international collaborations.6 During this period, initial evidence began emerging, from randomised trials, that population-based screening for CRC using gFOBT was effective in reducing CRC mortality and incidence, and improved tests emerged.2,6
In 1989, St John, Young and Macrae developed an initial set of guidelines that triggered extensive multidisciplinary consultation.2,7 The Australian Cancer Network, headed by Professor Tom Reeve, subsequently organised workshops and meetings to bring key experts (gastroenterologists, surgeons, public health specialists) together to discuss the potential for a national screening program. These initial guidelines formed a basis for the 1999 NHMRC-endorsed Australian Cancer Council (ACC) Guidelines for the prevention, early detection and management of colorectal cancer, which recommended population screening for CRC.
This recommendation was itself based upon key recommendations made in 1997 by the Australian Health Technology Advisory Committee (AHTAC). AHTAC was an Australian Government Department of Health committee established to provide advice on the cost-effectiveness of new and existing medical technologies.8 St John, Young and Salkeld were members of AHTAC’s CRC advisory team.
The guidelines played a central role in achieving a consensus among key organisations within the health system that a national screening program was needed and would be able to deliver a complete screening pathway from subject participation through detection and diagnosis to quality treatment.
However, in order to make such a program a reality, the screening method would need to be agreed upon, the public and the health profession would need to agree to participate, and the program would have to be shown to be cost effective.
Choosing the best test technology
The best test technology would, among other things, be the easiest for the public to use while also being sufficiently accurate for detecting neoplastic lesions, i.e. certain premalignant lesions and early-stage CRC.
Macrae and St John showed that bleeding from CRC varied from day to day, and precursor lesions (adenomas specifically) appeared to bleed only occasionally.9 This meant that a gFOBT-based approach would require three stool samples to be effective, which might not be broadly acceptable to the public. It was also clear that gFOBT technology had limited capacity to discriminate between those with and without CRC due to suboptimal sensitivity and diet-induced false positives.
It was clear that a more acceptable faecal test with improved clinical accuracy was needed, and Young and St John undertook a succession of studies to determine if there was a testing methodology superior to gFOBT.
A series of unique studies into the chemical fate of haemoglobin in the intestine showed that the haem and globin components suffered different chemical fates in the intestine.10,11 This had major implications for selective detection of bleeding from neoplastic lesions in the colorectum. gFOBT test results were affected by dietary factors, some drugs and vitamins. By contrast, Faecal Immunochemical Tests (FIT) for detecting globin removed the need for pre-test diet and drug restrictions.12 In addition, FIT were selective for colorectal bleeding, as proved by blood ingestion studies.13
In several major studies comparing the accuracy of several types of FIT to a standard and sensitive gFOBT, St John, Young, Macrae and colleagues demonstrated that FIT provided a better sensitivity for CRC without unacceptable problems with specificity.14,15 This was achieved with a 2-sample FIT compared to 3-sample gFOBT. They also demonstrated that FIT were capable of detecting adenomas.
Engaging the public and the profession
Participation in screening is a behavioural as well as a technological issue. For a national program to be a success, the screening test needed to be acceptable to invitees, and the support of a trusted advocate was desirable. When comparing gFOBT with FIT, Young and colleagues showed that removing dietary and drug restrictions, and reducing the sample number from three to two, significantly improved participation (by 70%).16
Providing invitees with a test kit accompanied by an endorsement from their local General Practitioner (GP) was found to be more credible than if the endorsement came from the screening program itself, and this approach improved participation by about 30%.17 Macrae, St John and colleagues had previously found that recognition by GPs of individuals at high risk for CRC, and GP knowledge about the proper use, follow up, and potential for CRC screening, all required emphasis in GP education programs.18
In an early (1984) study of people visiting their GP, Macrae and colleagues found the (prevailing) Health Belief Model to inadequately explain screening behaviour.19 Following that study, Young and colleagues demonstrated – in a population-based randomised controlled trial in Adelaide – that people were more likely to agree to be tested if they were provided information in an advance notification letter a few weeks prior to being offered the screening test.20 This strategy is now incorporated into the NBCSP and used in various countries around the world.
Cost effectiveness
Ultimately, the incorporation of population screening into the national health-care system would be dependent on a demonstration of cost-effectiveness, as a screening program would be associated with a wide range of costs. These would include producing and mailing-out test kits, testing the resultant samples, follow-up colonoscopies for those who tested positive, clinical examinations, removal of detected adenomas and cancer treatment (though the latter is less expensive for early-stage CRC).
In 1996, during the period leading up to the establishment of the AHTAC deliberations, Salkeld, Young and colleagues published research that modelled the cost-effectiveness of a screening program using Australian costs and CRC incidence, based on test accuracy from the Minnesota gFOBT trial.21 They showed that despite the relatively poor specificity of gFOBT, the cost per life-year-saved was $AUD24,660. This was of a similar magnitude to that of breast and cervical cancer screening and provided strong support for going forward with a national screening program.
Translation
The uncertainties relating to the most effective means of implementing a national CRC screening program led to a decision by government for there to be pilot and feasibility studies.
Through the Australian Government’s 2000-01 Budget, four years of funding was provided for a pilot program. This included funds to establish a Bowel Cancer Screening Pilot Register to identify who the participants in the pilot should be and to enable collection of relevant data about them.
In 2001, the Department of Health and Ageing established the Bowel Cancer Screening Pilot Implementation Committee (BCSPIC), with subcommittees chaired by Young and St John, to advise on the program’s design and implementation. The Committee’s Final Evaluation Report showed that a NBCSP would be feasible, acceptable, and cost-effective in Australia.22
A key decision made by the BCSPIC, based on research provided by the NHMRC-funded researchers, was to use FIT as the screening test rather than gFOBT, and to compare two brands of FIT in the pilot (based on the advice of Professor Les Irwig).
Funding was allocated to support the program in the 2006-07 Budget, and the program has continued and expanded since that time. From 2020, the NBCSP offered screening every two years to people aged 50-74 with expansion from 1 July 2024 to include those aged 45-49 years given the increasing incidence of CRC in that age group.
Subsequent studies have supported the NBCSP’s cost effectiveness. An important study from multiple colleagues including Jenkins and Canfell in 2018 modelled a range of alternative options for the NBCSP and concluded that the program in its current state is highly cost-effective and favourable compared to other alternatives.23

Aged Care
Outcomes and impacts
NHMRC-funded research and researchers have played a major role in establishing FIT as the preferred non-invasive CRC screening technology in use around the world.24 Australia was the first country to implement and sustain a unified national organised screening program using FIT.
Between the program’s commencement in Australia in August 2006 and 2023, over 10 million screening tests had been completed with about 4.5 million people participating at least once.25 The program has been contributing to reducing morbidity and mortality from CRC in Australia.26-28 For example, in 2009 it was reported that the Australian NBCSP diagnosed 3.2% of CRC cases, despite screening only being offered to people aged 55 or 65 years. These cases were diagnosed at an earlier stage compared to symptomatic cancer, indicating a likely significant impact on survival for these patients.28
As shown in Figure 3, rates of colon cancer for both women and men in Australia started to decline from the late 1980s.
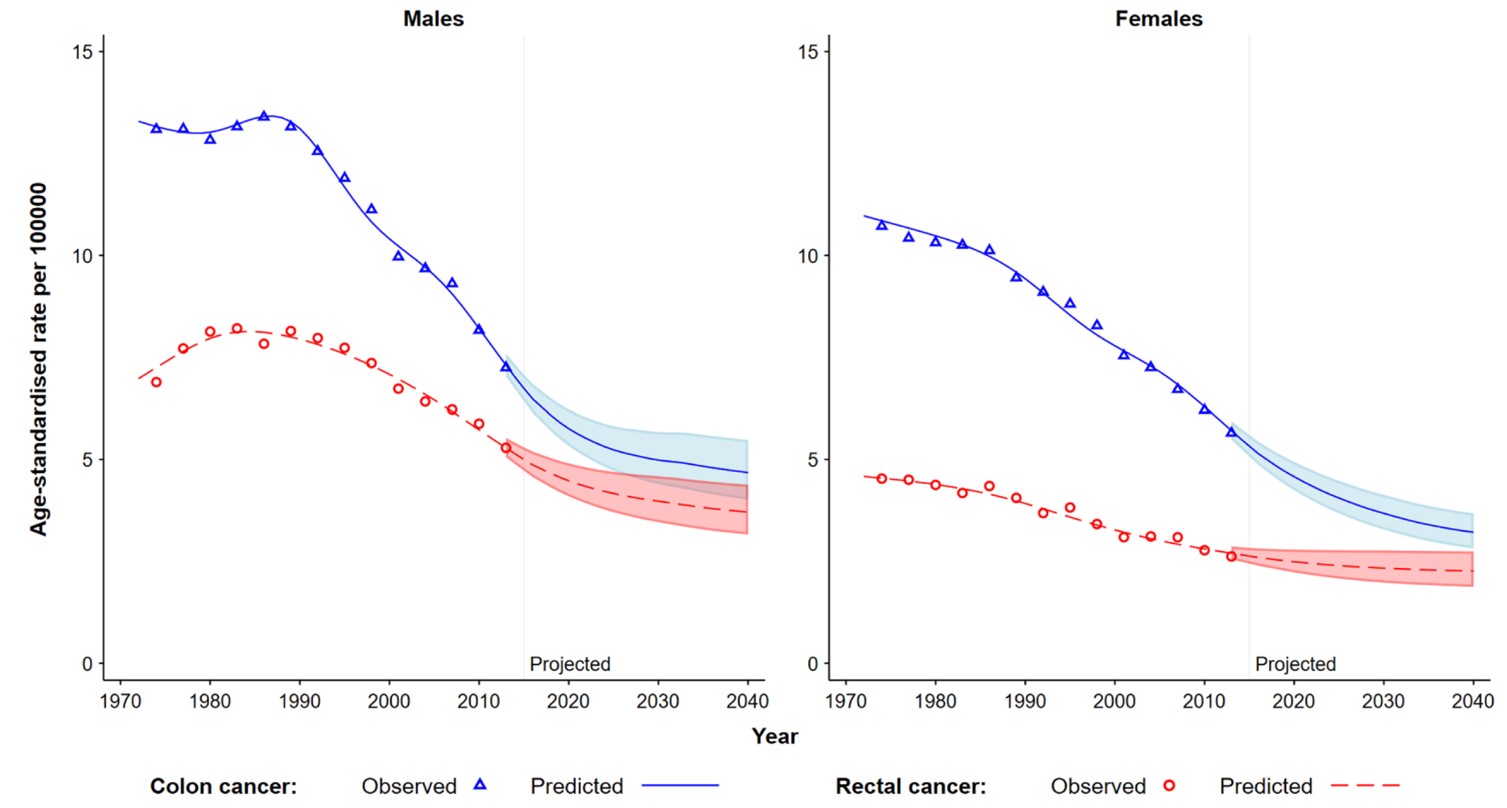
Nonetheless, in 2023, CRC was the second commonest cause of cancer-related death in Australia, second only to lung cancer, and caused a 50-70% higher rate of deaths than those due to prostate or breast cancer.25 Thus, CRC remains a major health burden on Australians. Further advances in FIT and other screening test technologies, and improvements to the population participation rate for the program, will be needed to reduce the incidence rate further.
Researchers
Professor D James B St John AO
Donald James Bourne St John graduated in medicine from the University of Melbourne in 1959. He received physician training in Australia and London, undertook academic and clinical roles at Monash University and the Alfred Hospital, and was then Director of the Department of Gastroenterology at the Royal Melbourne Hospital (RMH; 1977 to 2001). He is currently RMH Emeritus Consultant Gastroenterologist, Honorary Clinical Professorial Fellow, Department of Medicine at RMH, and Honorary Senior Associate, Cancer Council Victoria. He received The Royal Melbourne Hospital Award for Service to Health in 2001, the Cancer Council Victoria President's Award for Volunteer of the Year (2012), and was an inaugural inductee, The Royal Melbourne Hospital Research Hall of Fame (2014). He was made an Officer of the Order of Australia for service to medicine, medical research, as a gastroenterologist, to innovative public health cancer screening programs and as a mentor of young clinicians.
Professor Graeme P Young AM
Graeme Paul Young graduated from the University of Melbourne in 1969. He was previously Professor of Global Gastrointestinal Health at Flinders University and is currently Matthew Flinders Distinguished Emeritus Professor. He is a recipient of the Distinguished Research Prize of the Gastroenterological Society of Australia (2009), the Eureka Prize for Innovation in Medical Research (2017) and Fellow of the Australian Academy of Health and Medical Sciences and of Technological Science and Engineering. He became a Member of the Order of Australia in 2014 for services to medicine in the area of bowel cancer prevention. He is past chair of the World Endoscopy Association’s CRC Screening Committee.
Professor Finlay A Macrae AO
Finlay A Macrae completed medical training at Monash University in 1973. He is currently Head, Colorectal Medicine & Genetics, RMH, Professorial Fellow Department of Medicine, University of Melbourne, and was previously Deputy Director, Genomic Disorders Research Centre, Howard Florey Institute. He received the Distinguished Service Medal, Gastroenterological Society of Australia in 1999, Distinguished Research Prize of the Gastroenterological Society of Australia in 2021, and Master of the World Gastroenterological Organization. He was made an Officer of the Order of Australia for contributions to genetics and genomics internationally in 2016. He has also made major contributions to primary prevention of colorectal cancer through randomized controlled trials of diet and aspirin.
Professor Glenn P Salkeld
Glenn P Salkeld completed a PhD at University of Sydney in 2001. He is Honorary Professor of Public Health at the University of Sydney and University of Wollongong (UOW). From 2001 to 2016 he was a Chief Investigator (health economics) of the NHMRC Funded Program Grant on Screening and Diagnostic Test Evaluation and Co-Director of the Surgical Outcomes Research Centre (SOuRCe) at the Royal Prince Alfred Hospital. Glenn was Head of the University of Sydney School of Public Health for nearly a decade and later became Executive Dean of the Faculty of the Arts, Social Sciences and Humanities at UOW.
Other researchers
The impacts described in this case study were also the result of work undertaken by: Associate Professor Erin Symonds, Mr Stephen Cole, Professor Debra Turnbull, Professor Carlene Wilson, Professor Les Irwig, Professor Paul Glaziou, Professor Michael Solomon, Professor David Roder, Professor Karen Canfell, Professor Jon Emery and Professor Mark Jenkins.
Partner
This case study was developed with input from Professors Graeme Young, James St John, Finlay Macrae and Glenn Salkeld.
References
The information and images from which impact case studies are produced may be obtained from a number of sources including our case study partner, NHMRC’s internal records and publicly available materials. Key sources of information consulted for this case study include:
1 Standardised for age, refer: Australian Institute of Health and Welfare (2023) Cancer data in Australia, AIHW, Australian Government CAN 122. accessed 20 July 2024.
2 Young GP. Population-based screening for colorectal cancer: Australian research and implementation. Journal of Gastroenterology and Hepatology. 2009; 24 (S3): S33-42. PMID: 19799696
3 Federal Health Council. Report of the Federal Health Council of Australia. Tenth Session held at Perth, Western Australia, 21st to 24th September, 1936. Commonwealth of Australia, Canberra. p6
4 National Health and Medical Research Council. Report of the 67th Session of Council. Commonwealth of Australia, Canberra. p12
5 Greegor DH. Occult blood testing for detection of asymptomatic colon cancer. Cancer 1971; 28: 131–4.
6 Bresalier RS, Senore C, Young GP, et al. An efficient strategy for evaluating new non-invasive screening tests for colorectal cancer: the guiding principles. Gut, 2023; 72:1904-1918.
7 This work occurred under the auspices of the Australian Gastroenterology Institute
8 Department of Health Report of Director-General 1981-82.
9 Macrae FA, St. John DJB. Relationship between patterns of bleeding and Hemoccult sensitivity in patients with colorectal cancers or adenomas. Gastroenterology 1982; 82: 891–8.
10 Young GP, Rose IS, St John DJB. Hem in the gut. I: fate of hemoproteins and the absorption of hem. J. Gastroenterol. Hepatol. 1989; 4: 537–45.
11 Young GP, St John DJB, Rose IS, Blake D. Hem in the gut. Part II. Fecal excretion of hem and hem-derived porphyrins and their detection. J. Gastroenterol. Hepatol. 1990; 5: 194–203.
12 Fraser CG, Allison JE, Halloran SP, Young GP et al. A proposal to standardize reporting units for fecal immunochemical tests for hemoglobin. J Natl Cancer Inst. 2012;104:810–814
13 Young GP, Symonds EL, Allison JE, Cole SR, Fraser CG, Halloran SP, Kuipers EJ, Seaman HE. Advances in Fecal Occult Blood Tests: The FIT Revolution. Digestive Diseases and Sciences. 2015; 60: 609-622.
14 St John DJB, Young GP, Alexeyeff MA et al. Evaluation of new occult blood tests for detection of colorectal neoplasia. Gastroenterology 1993; 104: 1661–8.
15 Smith A, Young GP, Cole SR, Bampton P. Comparison of a brush-sampling fecal immunochemical test for hemoglobin with a sensitive guaiac-based fecal occult blood test in detection of colorectal neoplasia. Cancer 2006; 107: 2152–9.
16 Cole SR, Young GP, Esterman A, Cadd B, Morcom J. A randomized trial of the impact of new fecal hemoglobin test technologies on population participation in screening for colorectal cancer. Journal of Medical Screening, 2003; 10: 117-122.
17 Cole SR, Young GP, Byrne D, Guy J, Morcom J. Participation in faecal occult blood test-based screening for colorectal cancer is improved by endorsement by the primary care practitioner. Journal of Medical Screening, 2002;9:147-152.
18 Macrae FA, Hill DJ, Dent O, Goulston KJ, St John DJ. Colorectal cancer: knowledge and attitudes of doctors in Victoria. Aust. N. Z. J. Med. 1982; 12: 278–83.
19 Macrae FA, Hill DJ, St John DJ, Ambikapathy A, Garner JF. Predicting colon cancer screening behavior from health beliefs. Prev. Med. 1984; 13: 115–26.
20 Cole SR, Smith A, Wilson C, Turnbull D, Esterman A, Young GP. An advance notification letter increases participation in colorectal cancer screening. J. Med. Screen. 2007; 14: 73–5.
21 Salkeld S, Young G, Irwig L, Haas M, Glasziou P. Costeffectiveness analysis of screening by fecal occult blood testing for colorectal cancer in Australia. Aust. J. Public Health 1996; 20: 138–43.
22 Monitoring and Evaluation Steering Committee. Australia’s Bowel Cancer Screening Pilot and Beyond. Final Evaluation Report. Bowel Cancer Screening Pilot. Aust Government, Department of Health and Ageing. October 2005; Screening Monograph No. 6/2005
23 Lew JB, St. John DJB, Macrae FA, et al. Evaluation of the benefits, harms and cost-effectiveness of potential alternatives to iFOBT testing for colorectal cancer screening in Australia. Int. J. Cancer. 2018; 143:269–282
24 Young GP, Rabeneck L, Winawer SJ et al. The global paradigm shift in screening for colorectal cancer. Gastroenterology 2019;156:843-851
25 Refer https://www.aihw.gov.au/reports/cancer-screening/nbcsp-monitoring-2023/summary. See also Australian Institute of Health and Welfare 2021. Cancer in Australia 2021. Cancer series no. 133. Cat. no. CAN 144. Canberra: AIHW
26 Luo Q, Lew JB, Steinberg J, Worthington J, Yu XQ, Caruana M, Soerjomataram I, Bray F, Lawrance S, Arcorace M, O’Connell DL. Trends in colon and rectal cancer mortality in Australia from 1972 to 2015 and associated projections to 2040. Scientific Reports. 2022 Mar 7;12(1):3994
27 Cole SR, Tucker G, Byrne S, Osborne JM, Bampton P, Fraser RJ, Young GP. Shift to earlier stage at diagnosis as a consequence of the National Bowel Cancer Screening Program. Medical Journal of Australia, 2013 Apr 1;198(6):327-30
28 Ananda SS, McLaughlin SJ, Chen F, Hayes IP, Hunter AA, Skinner IJ, Steel MC, Jones IT, Hastie IA, Rieger NA, Shedda S. Initial impact of Australia's national bowel cancer screening program. Medical journal of Australia. 2009 Oct;191(7):378-81