The Australian Government is now in caretaker period. During this time, updates on this website will be published in accordance with the Guidance on Caretaker Conventions, until after the election.
Over the past century and throughout the world, viral hepatitis emerged as a significant public health issue afflicting hundreds of millions of people and causing severe ill health, liver damage, cancer and death.1
In collaboration with international colleagues, the National Health and Medical Research Council (NHMRC) funded researchers at The University of Western Australia, Fairfield Hospital's Epidemiological Research Unit (now the Burnet Institute) and Monash University made key contributions to the identification and control of viral hepatitis.
A landscape format version of this case study is available as a PDF (see the Downloads section below).
Origin
'Hepatitis' means liver inflammation and its health consequences may include fever, fatigue, nausea, vomiting, jaundice, cirrhosis of the liver and liver cancer. Hepatitis is most commonly caused by certain viruses. However, there are other causes including heavy alcohol use, some medications, toxins, other infections and autoimmune diseases.
Disease outbreaks resembling viral hepatitis have been known since ancient times2 and have especially occurred during wars.3 By the time of the Second World War, however, medical research had advanced to a stage that it was being conducted internationally on such outbreaks.
By 1946, the evidence indicated that there were at least 2 types of viral hepatitis.4 One of these was infectious and caused epidemics, had a short incubation period and was transmitted through ingestion of contaminated food or water. The other was most commonly transmitted through exposure to infected blood and had a longer incubation period. By 1953, the World Health Organization (WHO) had named these types (respectively) hepatitis A and hepatitis B (HB).5
In 1957, NHMRC recommended that hepatitis be proclaimed a notifiable disease in all states of Australia.6
A notifiable disease is required by law to be reported to government authorities, to allow authorities to monitor the disease and provide early warning of possible outbreaks.
However – and unlike other major infectious diseases – at this time the causative agent(s) for hepatitis had yet to be discovered7 and no blood tests for hepatitis were available.8 Medical research was required before progress could be made in preventing and controlling this disease.
Grants and investment
NHMRC-funded research at 2 different institutions contributed to international efforts to identify and control the hepatitis A and B viruses (HAV and HBV).
Commencing in 1950, Robert Kirk, a population geneticist at The University of Western Australia, received NHMRC grants to support basic research on blood proteins.
Also commencing in 1950, NHMRC funded a team of researchers based at the Epidemiological Research Unit of the Fairfield Infectious Diseases Hospital in Melbourne.
The unit included Alan Ferris, Ian Gust, Noreen Lehmann, Jacov Kaldor and Stephen Locarnini. Later, Ferris moved to Monash University and worked with the Fairfield team from there, along with electron microscopist Geoff Cross.
Results – hepatitis B
Kirk was investigating the different forms (polymorphisms) of various types of blood protein. The best known of these were the red blood cell antigens (that is, blood groups: A, B, AB, O) but Kirk was also interested in haptoglobins, transferrins, gamma globulins10 and lipoproteins.11
An antigen is a substance that generates an immune response.
This work was inherently collaborative – there were too many polymorphisms for a single researcher to investigate11 – and involved both other Australian11 and overseas researchers.10 Consequently, Kirk shared blood samples collected in Australia and South-East Asia8 with researchers at the US National Institutes of Health (NIH).11
Kirk had thought that his work might lead to an improved understanding of rheumatoid arthritis. However, in 1964, NIH researchers discovered that one of Kirk's samples contained a novel protein11 which, by 1968, had been confirmed to be the surface protein of HBV.13
This protein became known as 'Australia antigen' and its discovery provided a test for the presence of HBV. An immediate and important use for such a test was to confirm the safety of blood donations. The protein was also used to create the world’s first HBV vaccine, which was patented in 1972.14
The routine testing of blood donors to detect Australia antigen began recently ... Use of the test offers some promise of controlling the transmission of serum hepatitis through blood transfusion. If the test indicates the presence of Australia antigen, the blood concerned is rejected as unsuitable for use for blood transfusion purposes. Director-General of Health Annual Report 1970–7115
Results – hepatitis A
In 1965, when Gust commenced work at Fairfield Hospital, it was one of the largest infectious diseases hospitals in the world. Viral hepatitis was a very common disease in Melbourne at the time and, since Fairfield admitted 700 to 800 patients with the disease each year, identifying and treating hepatitis was a focus for research efforts there.16
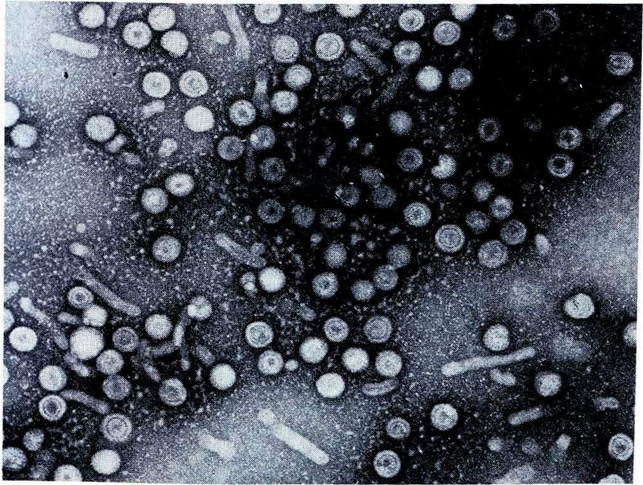
In 1969, equipped with samples of Australia antigen donated to Gust by NIH-funded groups, researchers at Fairfield began seeking to identify HAV, which they could now distinguish from HBV. Since HAV was known to be shed in faeces, they tested faecal extracts from patients with naturally acquired acute non-HB using blood serum that was thought to contain HAV antibodies. This test caused any HAV particles to cluster, making them visible when viewed through an electron microscope.16 Using this technique, in March 1974 they identified HAV, independently of but just after a team at NIH, who reported their findings in December 1973.5
An antibody is a blood protein that binds to a specific antigen.
In 1976, Gust joined the NIH team and brought with him faecal and blood samples selected by Lehmann from a group of Fairfield patients infected with HAV. The strain of HAV contained in one of these samples (code named HM175) was found to be particularly easy to work with. It was the first HAV strain to be isolated in cell cultures and sequenced and remains a standard HAV strain used in many laboratories today. Eventually, HM175 became the basis for the first commercial hepatitis A vaccine (Havrix™) which was licensed in 1991.16,17
In 1979, Locarnini and his colleagues reported the discovery of an HAV-specific antibody and a simple technique for the detection of HAV antibodies.18
Health outcomes and impact
Testing for Australia antigen quickly became routine in blood banks in many countries.14 The risk of acquiring hepatitis B from transfusion in Australia is now considered negligible, that is, less than 1 in 1 million per unit transfused.19 The test for antibodies developed by the Fairfield team remains the standard approach used internationally.20
Not only was the first-generation HB vaccine produced using Australia antigen but so are current vaccines. To prevent HBV infection, WHO recommends that all infants receive the HB vaccine as soon as possible after birth followed by 2 or 3 further doses to complete the vaccination series. Protection lasts at least 20 years and is probably lifelong.21
Vaccines for hepatitis A and B are both very effective and very safe.20,21 In those countries where these vaccines are widely available, these diseases have virtually disappeared.16
In 1983, Gust participated in a WHO Consultative Group that helped to shape the WHO Programme for the Control of Viral hepatitis.22 In 1986, Gust and 10 other international experts established an independent International Task Force on hepatitis B whose objective was to demonstrate that HB vaccination could be successfully added to WHO's Expanded Program on Immunization (EPI). The Task Force raised funding from a range of sources, including the Australian Government, to help it initiate 5 model immunisation programs – in Indonesia, Thailand, China, Kenya and Cameroon.23
The project in Lombok, Indonesia (1987–1991), was the first demonstration of the usefulness of HB vaccination in a developing country's immunisation program and contributed to the models used now by international aid organisations.24 As a consequence of the project, in 1992 the HB vaccine was recommended for inclusion in the WHO EPI.23
Timeline
| Year | Event |
|---|---|
| 1950 | Grants, Kirk (1950-1952); Grants, Ferris (1950-1960) |
| 1959 | Grants, Kirk (1959-1961) |
| 1964 | Grants, Ferris (1964,1967) |
| 1971 | Grants, Gust (incl. Ferris, Cross) (1971-1977) |
| 1973 | Grants, Gust/Lehmann (1973-1975) |
| 1974 | Grants, Ferris (1974-1976) |
| 1976 | Grants, Gust/Lehmann (1976,1979) |
| 1978 | Grants, Gust (1978-1980) |
| 1981 | Grants, Gust (1981-1983) |
| 1983 | Gust is WHO Hepatitis Programme consultant |
| 1986 | Gust partners to establish International Hepatitis B Taskforce |
| 1987 | Lombok, Indonesia Hepatitis B Model Immunization Project |
* Grants listed are NHMRC grants unless indicated otherwise.
Researcher profiles
Dr Robert Kirk
Robert Louis Kirk (1921–2010) was a chemist and zoologist at The University of Western Australia for over a decade, then became head of the Human Biology Department at the John Curtin School of Medical Research at The Australian National University (ANU, 1967–1987). In 1964, Kirk was appointed the national convenor of the International Biological Program – Human Adaptability study and was on the Australian Academy of Science subcommittee responsible for the study.
The blood samples that Kirk collected are a key resource managed by the ANU's National Centre for Indigenous Genomics.
Allan Ferris
Allan Aveling Ferris (1912–1997) was a member of the Australian Army Medical Corps during WWII. From 1940 to 1945, he was Officer-in-charge of the 104th Mobile Bacteriological Laboratory in New Guinea and later Pathologist to the 2/2 Australian General Hospital. He was Assistant Director of the Public Health Laboratory and Senior Lecturer in Microbiology at The University of Melbourne (1945–1948) then a pathologist and Director of the Epidemiological Research Unit at Fairfield Hospital (1948–1970) and reader in microbiology at Monash University (1970–1977). Ferris was President of the Australian Society for Microbiology (1974–1975).
Professor Ian Gust AO
Ian David Gust (1941-) studied medicine at the Universities of Melbourne and London, then became Director of the Virology Laboratory at Fairfield Hospital (1970–1990). Gust was Chief Commonwealth Medical and Scientific Adviser on AIDS and was the inaugural Director of the Macfarlane Burnet Centre for Medical Research (now the Burnet Institute). Gust was Director of Research and Development at CSL Ltd (1990–2000) and was President of the Australian Society of Microbiology (1998–2000). In 1992, Gust was appointed an Officer of the Order of Australia for service to public health, particularly in the prevention of hepatitis and AIDS.
Noreen Lehmann BSc
Noreen I Lehmann (1932-2020) graduated in science from The University of Melbourne in 1957 and in 1958 was recruited to join the Epidemiological Research Unit at Fairfield Hospital. At Fairfield, Lehmann was responsible for isolating a wide range of respiratory and enteric (intestinal) viruses as well as viruses responsible for childhood rashes. She was the first person in Australia to isolate rubella virus in cell culture and to establish a sensitive assay for rubella antibodies. She also undertook quality assurance of rubella tests performed by other laboratories.
Professor Stephen Locarnini AM
Stephen A Locarnini is Head of Unit, Research and Molecular Development (1998–2023) and Director Laboratory Services (1990–1998) at the Victorian Infectious Diseases Reference Laboratory (VIDRL). VIDRL was initially the Department of Pathology at Fairfield Hospital and is now part of the Peter Doherty Institute for Infection and Immunity. Locarnini is current Director of the WHO Collaborating Centre for Virus Reference and Research which is based at VIDRL.
In 2023, Locarnini was appointed a Member of the Order of Australia for significant service to medicine as a virologist, and to medical research.
Dr Geoff Cross
Geoff Cross was an electron microscopist at Monash University.
Dr Jacov Kaldor
Jacov Kaldor was Senior Biochemist within the Biochemistry Department at Fairfield Hospital.
Professor Ron Lucas
Charles Ronald Lucas (1932–2009) graduated in Medicine from The University of Melbourne, then commenced work as a physician at Fairfield Hospital in 1964, where he remained until his retirement in the early 1990s.
References
This case study was developed with input from Professor Ian Gust and in partnership with the Burnet Institute, The University of Western Australia and Monash University.
The information and images from which NHMRC Impact Case Studies are produced may be obtained from sources including our case study partner, NHMRC's internal records and publicly available materials.
The following sources were consulted for this case study:
- Division of Viral Hepatitis, National Center for HIV, Viral Hepatitis, STD, and TB Prevention. Viral Hepatitis. Centres for Disease Control and Prevention. Atlanta, United States. 19 July 2021.
- Thomas E, Yoneda M, Schiff ER. Viral hepatitis: past and future of HBV and HDV. Cold Spring Harbor Perspectives in Medicine. 2015; 5(2):a021345
- Trepo C. A brief history of hepatitis milestones. Liver International. 2014; 34:29-37
- Maccallum FO. Homologous Serum Hepatitis. Proceedings of the Royal Society of Medicine. 1946; 39(10):655-7
- Feinstone SM. History of the discovery of the hepatitis A virus. Cold Spring Harbour Perspectives in Medicine 2019; 9:a031740
- National Health and Medical Research Council. Report of the 43rd Session of the National Health and Medical Research Council. Australia: NHMRC; May 1957, Volume 1, p16
- Other types of hepatitis viruses have subsequently been identified: hepatitis C (1989), hepatitis D (1977) and hepatitis E (1978).
- National Health and Medical Research Council. National Health and Medical Research Council: Report for 1970. Parliamentary Paper No. 122. The Parliament of the Commonwealth of Australia: 1971, p203
- National Health and Medical Research Council. National Health and Medical Research Council: Report for 1971. Parliamentary Paper No. 242. The Parliament of the Commonwealth of Australia: 1972, p180
- National Health and Medical Research Council. Report of the 51st Session of the National Health and Medical Research Council. Australia: NHMRC; May 1961, Volume 1, p17
- Blumberg BS. Hepatitis B—The hunt for a killer virus. Princeton University Press. 2003. p79
- National Health and Medical Research Council. Report upon the work done under the Medical Research Endowment Act during the year 1959. Commonwealth of Australia: 1960, pp16-17
- Gust ID. Recent developments in hepatitis A. Pathology. 1978; 10(4):299-306
- Blumberg BS. Australia antigen and the biology of hepatitis B. Science. 1977; 197(4298):17-25
- Director-General of Health. Annual Report for year 1970-71. Parliamentary Paper No. 191. The Parliament of The Commonwealth of Australia. 1971, p92
- Gust ID. Viral hepatitis: Some reflections on a personal journey. Human Vaccines. 2009; 5(5):357-360
- Gust I, Kennett M. Generous mentor to two generations of scientists and physicians. The Sydney Morning Herald. Obituaries. Noreen Lehmann 1932-2020. 15 June 2020
- Locarnini SA, Coulepis AG, Stratton AM, Kaldor JA, Gust ID. Solid-phase enzyme-linked immunosorbent assay for detection of hepatitis A-specific immunoglobulin M. Journal of Clinical Microbiology. 1979; 9(4):459-65
- Australian Red Cross Lifeblood. Hep B transfusion risk lower. 19 January 2017.
- World Health Organization. Hepatitis A fact sheet. 24 June 2022.
- World Health Organization. Hepatitis B fact sheet. 24 June 2022.
- National Health and Medical Research Council. Report of the Ninety-sixth session: 26-27 October 1983. NHMRC: Canberra, October 1983, p504
- Ruff TA, Muller N, Gust ID. Hepatitis B control: lessons from the International Task Force on Hepatitis B Immunization and the Lombok Hepatitis B Model Immunization Project. Progress in Liver Diseases. 1993; 11:179-201
- Van Der Weyden MB. Bench‐to‐bedside research in Australian research institutes: a snapshot. Medical Journal of Australia. 2003; 179(11):603-10
Partners





