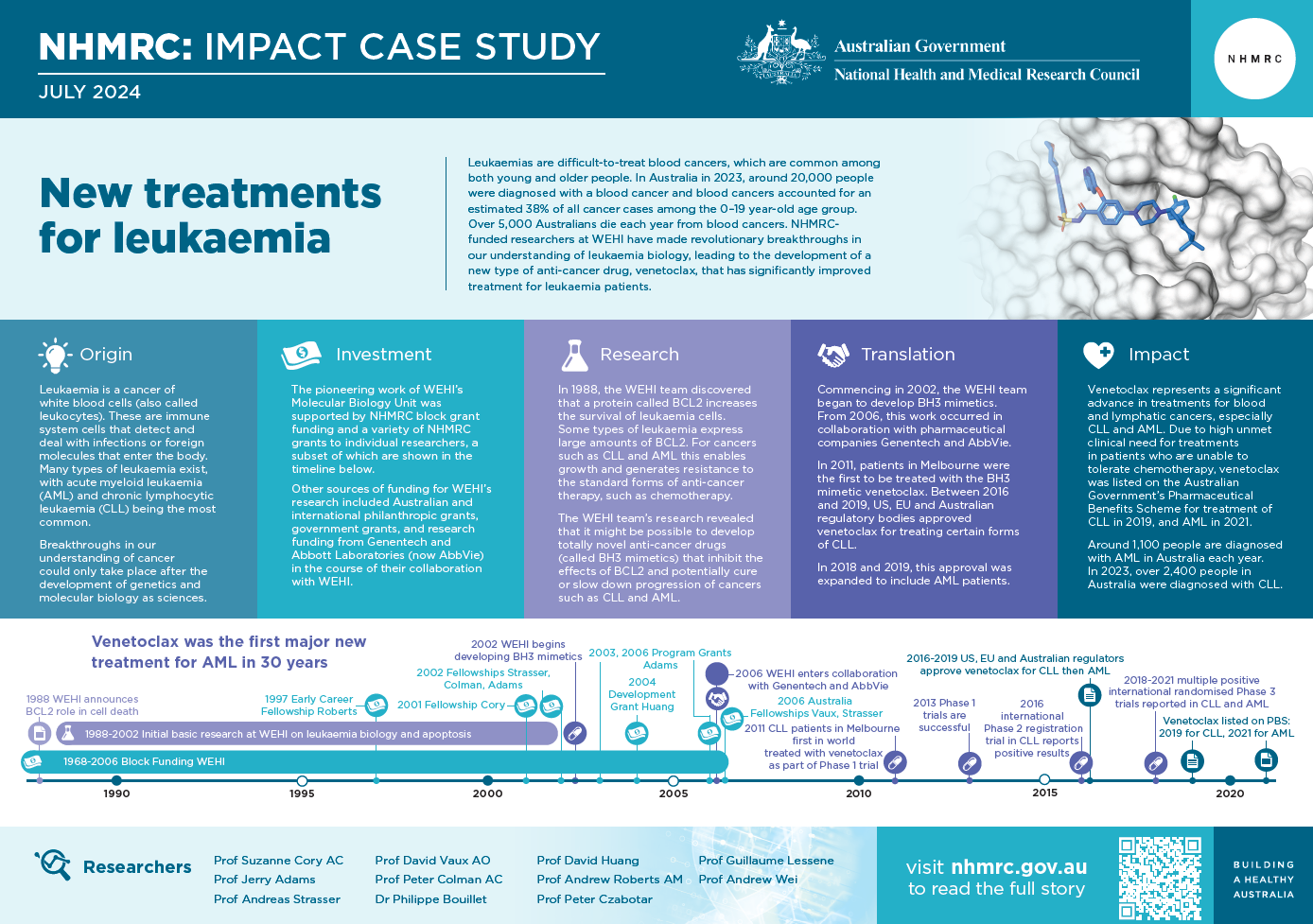
Leukaemias are difficult-to-treat blood cancers, which are common among both young and older people. In Australia in 2023, around 20,000 people were diagnosed with a blood cancer and blood cancers accounted for an estimated 38% of all cancer cases among the 0–19 year-old age group. Over 5,000 Australians die each year from blood cancers.1 NHMRC-funded researchers at WEHI have made revolutionary breakthroughs in our understanding of leukaemia biology, leading to the development of a new type of anti-cancer drug, venetoclax, that has significantly improved treatment for leukaemia patients.
Origin
Leukaemia is a cancer of white blood cells (also called leukocytes). These are immune system cells that detect and deal with infections or foreign molecules that enter the body. Many types of leukaemia exist, with acute myeloid leukaemia (AML) and chronic lymphocytic leukaemia (CLL) being the most common.
Because cancer is ultimately a genetic disease, key breakthroughs in our understanding of cancer could only take place after the development of the science of molecular biology, which investigates the biochemical and genetic bases of living systems.
A major milestone for molecular biology was the development of the double helix model of DNA. This took place in 1953 in the UK, in what became the Medical Research Council Laboratory of Molecular Biology (LMB).
In 1971, when LMB researchers Suzanne Cory and Jerry Adams arrived from the UK to establish Australia’s first molecular biology laboratory at WEHI, the focus of the institute’s research was immunology. Gaining a better understanding of cancer genetics in the immune system was to be a focus of their efforts.
Investment
The pioneering work of WEHI’s Molecular Biology Unit was initially supported by NHMRC block grant funding to WEHI and later by a variety of other NHMRC grants, including program grants and fellowships to individual researchers.
Key members of the team included Suzanne Cory, Jerry Adams, Andreas Strasser, Peter Colman, David Huang, David Vaux, Philippe Bouillet, Andrew Roberts, Guillaume Lessene, Peter Czabotar and Andrew Wei.
Other sources of funding for their research included Australian and international philanthropic grants, government grants, and research funding from Genentech and Abbott Laboratories (now AbbVie) in the course of their collaboration with WEHI.
The PDF poster version of this case study includes a graphical timeline showing the NHMRC grants provided and other events described in the case study.
Research
Inspired by the 1976 discovery of genes (called oncogenes) that when mutated can play a role in the formation of cancer, the WEHI team investigated oncogene involvement in leukaemias and tumours of the immune system. In the process, team member David Vaux discovered (and reported in 1988) the function of a new oncogene – called B cell lymphoma 2 or BCL2 – that produces a protein that improves the survival of leukaemia cells.
The WEHI team had made a major discovery as this was the first time prolonged cell survival had been suggested as a cause for cancer. The BCL2 protein was the first apoptosis (cell death) regulator identified in any organism.2
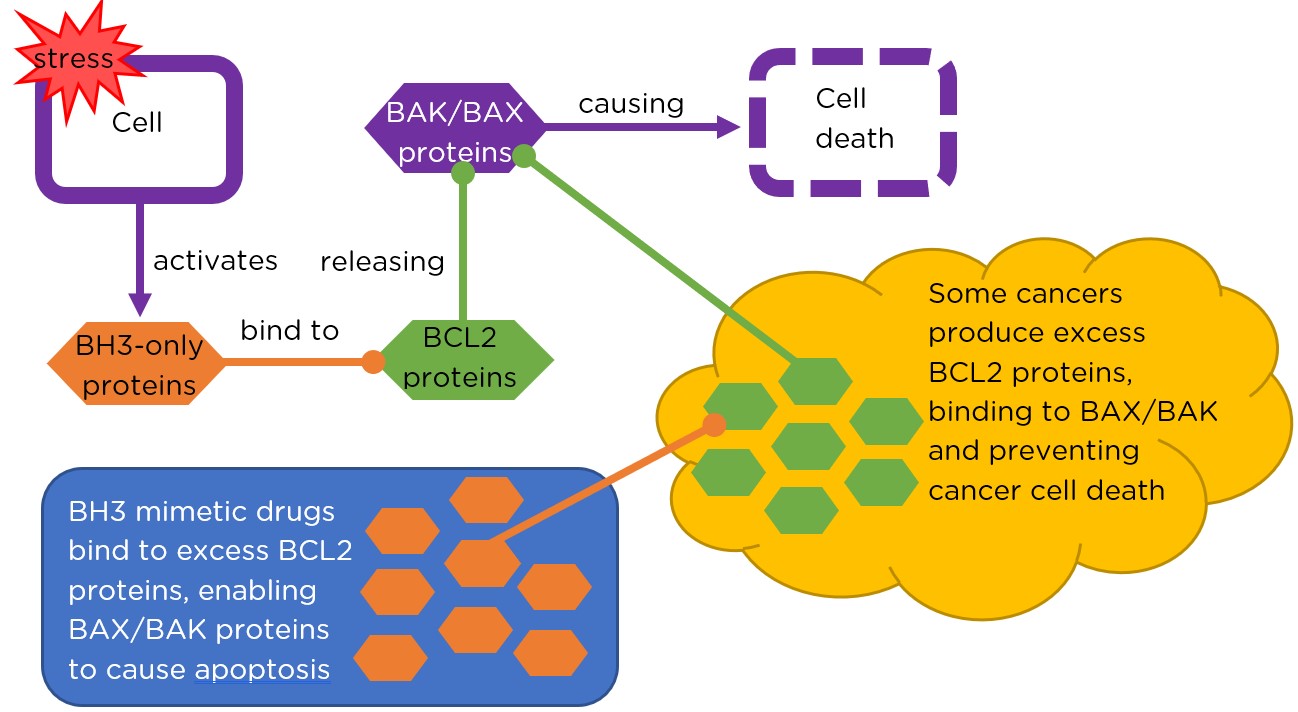
Apoptosis occurs when a cell under stress releases special ‘demolition’ proteins called BAX and BAK. Normally, these proteins are bound to BCL2 proteins and are unable to cause damage. However, if another type of protein (called ‘BH3-only’) is released in a cell, it binds to the BCL2 proteins where BAX and BAK bind. This releases BAX and BAK which act together to instigate the cell’s death.
Some types of leukaemia, such as CLL and AML, express large amounts of BCL2 protein, preventing the release of BAK and BAX and thus apoptosis, thereby prolonging leukaemia cell survival. For cancers such as CLL and AML this enables growth and generates resistance to the standard forms of anti-cancer therapy, such as chemotherapy.
The WEHI team’s research revealed that it might be possible to develop totally novel anti-cancer drugs that mimic the effect of BH3-only proteins (called BH3 mimetics), curing or slowing down the progression of cancers such as CLL and AML.
Translation
Commencing in 2002, the WEHI team began the process to design a BH3 mimetic drug. They looked through WEHI’s smart chemical library – containing over 110,000 compounds – and found a single candidate class (called benzothiazoles) that possessed a similar shape to BH3 proteins.
In 2006, WEHI entered into a collaboration agreement with pharmaceutical company Genentech for the discovery, development and commercialisation of BH3 mimetics for the treatment of cancer. A year later, another pharmaceutical company, Abbott Laboratories (now AbbVie), which had independently developed its own BH3 mimetic, entered a three-way collaboration with WEHI and Genentech to develop an anti-cancer drug.
In 2009, laboratory testing of the drug candidate, that would ultimately be developed into venetoclax, revealed its promise in treating cancers with high levels of BCL2. WEHI researchers were the first to test the drug on leukaemia cells obtained from patients.
In 2011, CLL patients in Melbourne at The Royal Melbourne Hospital and Peter MacCallum Cancer Centre were the first in the world to be treated with venetoclax in Phase I clinical trials. By 2013, the Phase I trials had shown promising results for treating advanced CLL. Venetoclax was found to lower leukaemia cell numbers in patients for whom conventional treatment options (such as intensive chemotherapy) had failed or were not available.
Final results of the Phase I study were released in December 2015 and confirmed the potency of venetoclax. Twenty per cent of patients treated with venetoclax in these trials achieved complete clearance of the cancer and 54 per cent showed partial clearance.
In 2016, Phase II clinical trials conducted internationally, including in Australia, demonstrated that venetoclax was effective for treating advanced forms of CLL. Of the people involved in the Phase II trial, 79% gained some benefit from it, with most trial patients showing substantial reductions in the number of leukaemia cells in their body. Updated results of the Phase I study also confirmed that these responses could be lasting.
Between 2016 and 2019, US, EU and Australian drug regulatory bodies approved venetoclax for treating CLL, enabling doctors to prescribe venetoclax to patients with this blood cancer. In 2018 and 2019, this approval was expanded to include AML patients unsuitable for intensive chemotherapy.
One of the key advantages to venetoclax is its high specificity for BCL2 over other similar proteins. This specificity makes an important contribution to the drug’s safety as it helps to ensure that leukaemia treatment using venetoclax does not cause harm by disrupting any of the body’s other biochemical processes that involve BCL2-family proteins.

Outcomes and impacts
Venetoclax represents a significant advance in treatments for these blood cancers. The impact is great in both CLL and AML, but especially important in AML where treatment has not otherwise advanced appreciably during the past 30 years.4 In the context of high unmet clinical need for treatments in patients who are unable to tolerate the existing standard of care for CLL, venetoclax was listed on the Australian Government’s Pharmaceutical Benefits Scheme in 2019. The listing now includes previously untreated patients with CLL and was extended to include treatment for AML in 2021. Around 1,100 people are diagnosed with AML in Australia each year.5 In 2023, over 2,400 people were diagnosed with CLL.6
In combination with other drugs, and particularly in cases where a cancer may be resistant to chemotherapy because of its high levels of BCL2 expression, venetoclax has led to improved outcomes and has become a standard of care for frontline treatment in older patients or those unable to undergo intensive chemotherapy.
Studies have demonstrated that patients with CLL and AML have experienced strong positive outcomes when using venetoclax (especially in combination with other medications), including a higher percentage of patients having their cancer cells partially or fully cleared following treatment, and increased progression-free survival.
Venetoclax is currently being tested in clinical trials to determine its potential benefits for other blood cancers and patient groups, and WEHI’s work has also prompted the investigation of a range of new BH3 mimetics and BCL2 inhibitors, some that are now in early clinical trials.
Researchers
Professor Suzanne Cory AC
Suzanne Cory received her undergraduate degree in biochemistry from The University of Melbourne, then in 1966 commenced a PhD at the MRC Laboratory of Molecular Biology in Cambridge, UK. After working as a postdoctoral researcher in Geneva, Cory returned to Melbourne on a Queen Elizabeth II Fellowship and jointly established molecular biology at WEHI in 1971 with Jerry Adams. Cory became director of WEHI and Professor of Medical Biology of The University of Melbourne (1996-2009) and President of the Australian Academy of Science (2010-2014). She was appointed a Companion of the Order of Australia in 1999.
Professor Jerry Adams
Jerry McKee Adams studied science at Emory University in Atlanta then completed a PhD at Harvard University. Adams was awarded a Helen Hay Whitney Fellowship to pursue postdoctoral training at the LMB, then moved to the Institut de Biologie Moléculaire, at the University of Geneva, and then to WEHI. Adams was awarded the Centenary Medal in 2001.
Professor Andreas Strasser
Andreas Strasser completed a Masters degree and PhD at the Basel Institute for Immunology and the University of Basel in Switzerland, before joining WEHI as a postdoctoral fellow in 1989, then laboratory head. Strasser is head of the Blood Cells and Blood Cancers division at WEHI.
Professor David Vaux AO
David Laurence Vaux studied medicine at The University of Melbourne and then completed a PhD at WEHI. After working as a postdoctoral researcher at Stanford University, Vaux returned to WEHI where he is now an Honorary Research Fellow. Vaux was appointed an Officer of the Order of Australia in 2017.
Professor Peter Colman AC
Peter Malcolm Colman completed a PhD in physics at the University of Adelaide in 1969. He was a postdoctoral fellow at the University of Oregon (1969-1972) and at the Max Planck Institute, Munich (1972-1975) and a Queen Elizabeth II fellow at The University of Sydney (1975-1978). Colman joined CSIRO’s Division of Protein Chemistry in 1978, was appointed Foundation Chief of the CSIRO Division of Biomolecular Engineering in 1989, then established the Structural Biology division at WEHI in 2001. Colman was appointed a Companion of the Order of Australia in 2017.
Dr Philippe Bouillet
Philippe Bouillet completed a PhD at the Institute for Genetics and Cellular and Molecular Biology in Strasbourg, France then in 1997 joined WEHI to work in the Molecular Genetics of Cancer division. Bouillet’s research was supported by a 2002 Viertel Senior Medical Research Fellowship. He is currently an Honorary Research Fellow in WEHI’s Inflammation division.
Professor David Huang
David Huang undertook medical training in London followed by post-graduate clinical training and a PhD on oncogenic signalling. Huang joined WEHI in 1994 as a Leukaemia Lymphoma Society Fellow, then (as a Viertel Fellow) established his own laboratory at WEHI in the Blood Cells and Blood Cancers division. Huang is also Professor in the Department of Medical Biology at The University of Melbourne. Huang, along with Peter Czabotar, Guillaume Lessene and Andrew Roberts was awarded the 2019 Prime Minister’s Prize for Innovation.
Professor Andrew Roberts AM
Andrew Warwick Roberts received training in haematology at the Royal Brisbane Hospital then undertook a PhD at WEHI. After post-doctoral research at Indiana University, he returned to Australia and is now Metcalf Chair in Leukaemia Research at the Centre for Cancer Research at The University of Melbourne. Roberts also jointly leads WEHI’s Cancer Research and Treatments theme and is a clinical haematologist at the Royal Melbourne Hospital and Peter MacCallum Cancer Centre. Roberts was appointed a Member of the Order of Australia in 2020.
Professor Peter Czabotar
Peter E Czabotar undertook his PhD and worked at Curtin University, the Telethon Kids Institute and the National Institute of Medical Research in London before joining WEHI. Czabotar is joint head of WEHI’s Structural Biology division.
Professor Guillaume Lessene
Guillaume Lessene trained as an organic chemist, completing his PhD at the University of Bordeaux before undertaking postdoctoral work at Pennsylvania State University. Lessene moved to WEHI in 2001, becoming a lab head in 2009. Since 2019, he has led the Institute’s New Medicines and Advanced Technologies theme.
Professor Andrew Wei
Andrew H Wei completed his medical degree in 1993 then specialised as a clinical haematologist, doing a PhD at WEHI. He is Co-Stream lead for acute leukaemia and myelodysplastic syndromes at the Peter MacCallum Cancer Centre and the Royal Melbourne Hospital and leads multiple AML clinical trials through the Australasian Leukaemia and Lymphoma Group. Wei holds a joint appointment as the Metcalf Family Fellow and a laboratory head in the Blood Cells and Blood Cancers division at WEHI.
Partner
This case study was prepared with input from Professor Andrew Roberts and colleagues and in partnership with WEHI.

References
The information and images from which impact case studies are produced may be obtained from a number of sources including our case study partner, NHMRC’s internal records and publicly available materials. Key sources of information consulted for this case study include:
1 Australian Institute of Health and Welfare. Cancer data in Australia web report. Cat. No: CAN 122. AIHW 2024
2 Kelly GL, Strasser A. Toward targeting antiapoptotic MCL-1 for cancer therapy. Annual Review of Cancer Biology. 2020;4:299-313
3 Birkinshaw RW, Gong J, Luo CS, Lio D, White CA, Anderson MA, Blombery P, Lessene G, Majewski I, Thijssen R, Roberts AW, Huang DCS, Colman PM, Czabotar PE. Structures of BCL-2 in complex with venetoclax reveal the molecular basis of resistance mutations. Nature Comms 2019; 10(1):2385
4 Lachowiez C, DiNardo CD, Konopleva M. Venetoclax in acute myeloid leukemia - current and future directions. Leuk Lymphoma. 2020;61(6):1313-1322
5 Department of Health and Aged Care. New treatment for Australians with leukaemia [Internet]: Department of Health and Aged Care; 2021 [published 28 November 2021; cited 9 April 2024]. Available from https://www.health.gov.au/ministers/the-hon-greg-hunt-mp/media/new-treatment-for-australians-with-leukaemia
6 Australian Institute of Health and Welfare. Cancer data in Australia, Cancer rankings data visualisation - Australian Institute of Health and Welfare (aihw.gov.au). Accessed 1 July 2024.