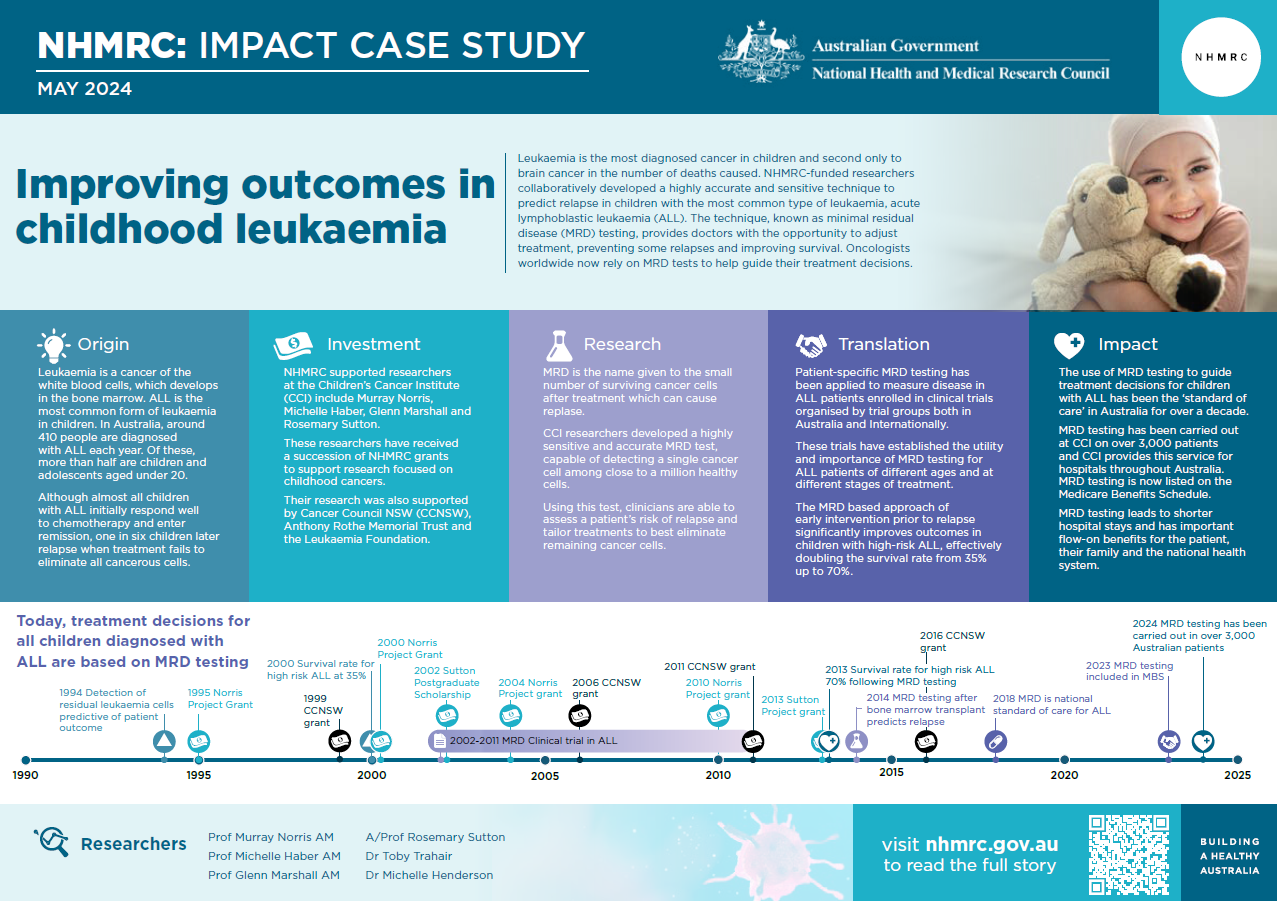
Leukaemia is the most diagnosed cancer in children and the second most common cancer causing death among children in Australia.1 NHMRC-funded researchers at the Children’s Cancer Institute (CCI), in collaboration with researchers at Flinders University and in European laboratories, developed a highly accurate and sensitive technique – known as minimal residual disease (MRD) testing – that enables doctors to improve anti-cancer treatment for children with the most common type of leukaemia.
Origin
Cancer is a group of diseases characterised by the uncontrolled division of abnormal cells and their spread to nearby tissue.2
During the 1960s, the survival rate for children with cancer was 30%.3 Since that time, research-based medical advancements and technological improvements in cancer diagnosis and treatment have allowed the average survival rate across the different types of childhood cancer to reach 80%.1 Nonetheless, certain types of cancers remain a serious problem for children.
Leukaemia is a cancer of the white blood cells and develops in the bone marrow. There are a variety of types of leukaemia, and these are separated by how quickly they grow (acute or chronic) and the specific white blood cells affected. These cancers can occur in both adults and children, with leukaemia representing the most common form of childhood cancer.
Acute lymphoblastic leukaemia (ALL) is the most common form of leukaemia in children. In Australia, around 410 people are diagnosed with ALL each year. Of these, more than half are children and adolescents aged under 20.4 Other forms of leukaemia – which include chronic lymphocytic leukaemia (CLL), acute myeloid leukaemia (AML) and chronic myeloid leukaemia (CML) – occur more frequently in adults.
While almost all children with ALL initially respond well to chemotherapy and enter remission, for one in six children, treatment fails to eliminate all the leukaemia cells. In these cases, the very small number of remaining cells (known as MRD) can lead to cancer relapse, often with resistance to the initial treatment and a significantly increased likelihood of mortality caused by the cancer.
Investment
CCI researchers Murray Norris, Michelle Haber, Glenn Marshall and Rosemary Sutton have received a succession of NHMRC grants to support research focused on childhood cancers, including ALL and neuroblastoma (cancer of the nerve tissue), and particularly with respect to:
- leukaemia treatment and risk evaluation
- how to improve survival rates and reduce relapse and long-term side effects in recovered cancer patients
- molecular therapies to delay cancer growth, novel drug combinations and approaches to control drug resistance
- exploring technologies to identify combination drug therapies and strategies for cancer prevention.
The work of these CCI researchers was also supported by Cancer Council NSW, Anthony Rothe Memorial Trust and the Leukaemia Foundation.
The PDF poster version of this case study includes a graphical timeline showing NHMRC grants awarded and other events described in the case study.
Research
In 1984, Norris and Haber began working at CCI’s newly opened research laboratories, located within the campus of Sydney Children’s Hospital and adjacent to the University of New South Wales (UNSW). Their work soon began to include a focus on drug resistance in leukaemia cells.
Over time, and in close collaboration with their clinical colleague Glenn Marshall (at the Kids Cancer Centre, also based at the hospital), they set about developing a technique for detecting and measuring MRD at a molecular level, so that children with high levels of MRD could be reliably identified before relapsing. This technique needed to be both sensitive enough to detect miniscule quantities of leukaemia cells, and rapid enough to provide immediate feedback to clinicians on treatment effectiveness.
Having identified unique genetic markers for each patient’s leukaemia, the CCI researchers then found a way to measure very low levels of these markers in samples collected from each patient’s bone marrow, at different stages of treatment. In retrospective studies performed on patient leukaemia samples, the team was able to show that MRD testing was highly predictive of patient outcomes. However, to be used in clinical practice, the tests needed to be able to provide results quickly so that clinicians could adjust each patient’s therapy in time to prevent relapses.
Further refinement of the technology led to MRD tests that could simultaneously detect and quantify the level of residual leukaemia present in a patient’s bone marrow and return the result within two weeks.
The first step in MRD testing is to analyse the leukaemia cells in a sample of the patient’s bone marrow at diagnosis. Using DNA sequencing and other molecular techniques, unique genetic signatures of these leukaemia cells are identified, to serve as ‘markers’ for the detection of leukaemia at a later time. After the patient has received treatment, testing can then be done to detect and quantify these markers, showing the level of leukaemia left in the patient’s body.
The MRD test system they developed is highly sensitive, capable of detecting a single cancer cell among a million healthy cells.5 It is also accurate, allowing clinicians to reliably assess a patient’s risk of relapse and tailor their treatment appropriately to eliminate the remaining cancer cells, with the intention of preventing relapse.
Translation
The first Australian ALL clinical trial to test MRD was conducted by the Australian and New Zealand Children's Haematology/Oncology Group (ANZCHOG) of children’s oncologists and enrolled over 600 patients from 2002 -2011. A team of scientists at CCI led by Sutton performed all the MRD tests, and those children identified by MRD testing to be at high risk of relapse were given more intensive chemotherapy and bone marrow transplantation by their treating clinicians. This approach of early intervention prior to relapse significantly improved outcomes in children with high-risk ALL, effectively doubling the survival rate from 35% up to 70%.6
This success was followed with the evaluation of MRD in children with relapsed ALL from the United Kingdom and Australia. The evaluation found that MRD-guided treatment significantly improved patient survival while at the same time avoiding transplantation in children with good MRD test results.6 Further studies demonstrated that the use of MRD diagnostics was feasible and useful in: babies with MLL-ALL, a special subtype of ALL; children receiving bone marrow transplants;7 and in ALL trials for younger adults and older adults.8
The CCI team applied patient-specific MRD tests in real-time to measure disease in ALL patients enrolled in clinical trials organised by co-operative trial groups both in Australia and internationally. These trials established the utility and importance of MRD testing for ALL patients of different ages (children, adolescents and young adults, older adults and babies) at different stages of treatment (newly diagnosed, later in treatment, after relapse and before or after transplant). Usually, at least one aspect of treatment was defined by how well the patient responded to therapy as measured by MRD.
Based on the evidence supporting its value and on a quality assurance program developed in collaboration with the EuroMRD labs, CCI researchers worked with the Royal College of Pathologists of Australasia to gain approval for Medicare funding to be available for molecular MRD testing.
Outcomes and impacts
The use of MRD testing to guide treatment decisions for children with ALL has now been the ‘standard of care’ in Australia for over a decade.9
As a consequence of the introduction of MRD in Australia, between 1986–1991 and 2010–2015 the 5-year relative survival for children aged 0–14 for all leukaemias increased from 70% to 90%. By comparison, in the same period the five-year relative survival rate for childhood cancer overall improved from 71% (for children diagnosed between 1983-92) to 84% (diagnosed between 2003-2012).1
Since the development of the MRD technology, scientists in CCI’s MRD Group have carried out testing on over 3,000 patients and they provide this service for hospitals throughout Australia. MRD testing is used to determine future risk of relapse, as well as to monitor for the first signs of relapse in patients known to be at high risk. Over time, CCI researchers have refined their techniques, and it is now possible to use the test to predict whether standard treatment is likely to cure a child within the first month of their treatment.
As well as showing which children are at high risk of relapse, MRD testing also shows which children are at lower risk of relapse and therefore can be given less intensive treatment. This helps avoid unnecessarily harsh treatment and side effects in these children such as bone marrow transplantation, limiting their health risks.
In 2023, the Australian Government’s Medical Services Advisory Committee (MSAC) supported registration of MRD as a Medicare item. MSAC noted that patients whose MRD testing led them to receive reduced intensification/maintenance chemotherapy experienced less infection-related ill health, fewer outpatient episodes of fever and of shorter duration, a lower proportion of fever episodes resulting in hospitalisation and intensive care unit admission, less chemotherapy interruptions, fewer intravenous antibiotic courses and shorter hospital stays.
Because spending less time in hospital also means a reduced impact on the entire family and lower indirect costs of treatment, use of MRD has important flow-on benefits for the patient and their family, as well as for the national health system.
The health benefits arising from MRD research don’t just relate to children – MRD has also been broadly adopted in adult ALL practice as well. This has included using MRD testing:
- as part of adult clinical trials
- to classify adult ALL patients into lower versus higher risk of relapse groups and to then allocate them to low risk (chemotherapy only) versus high-risk therapy (stem cell transplantation)
- for eligibility under the Australian Government’s Pharmaceutical Benefits Scheme to receive blinatumomab, an immunotherapy treatment that makes it easier for the immune system to recognise leukaemia cells.
CCI’s researchers have also shown that whole-genome sequencing data can be used to design patient-specific MRD assays, not just for ALL but also for other childhood cancers, thus extending MRD testing to solid tumours such as neuroblastoma and sarcoma. Over time, CCI’s continuing research is expected to both extend the use of MRD and to reduce its costs.
Researchers
Professor Murray Norris AM
Murray David Norris graduated from the Australian National University in 1977 with a Bachelor of Science (Biochemistry) and was one of three scientists appointed to CCI when it first opened in 1984. He completed a PhD at UNSW in 1988. Norris served as CCI Deputy Director (2000-2023) and Director of the UNSW Centre for Childhood Cancer Research (2015-2023), and currently leads CCI’s Molecular Oncology Group. Norris was appointed a Member of the Order of Australia in 2015.
Professor Michelle Haber AM
Michelle Haber completed a PhD at UNSW’s School of Pathology in 1984 and was then appointed the inaugural post-doctoral scientist at CCI. She became Director of the Institute in 2000, has been Executive Director since 2003 and also leads the Institute’s Experimental Therapeutics Group. Haber is an inaugural Fellow of the Australian Academy of Health and Medical Sciences (2015) and a Fellow of the Australian Academy of Science (2022) and was appointed a Member of the Order of Australia in 2007.
Professor Glenn Marshall AM
Glenn M Marshall is a clinician whose primary appointment has been as a Paediatric Oncologist at the Kid’s Cancer Centre. He has also served as the Head of Translational Research at CCI, was Clinical Lead of CCI’s Zero Childhood Cancer Program and currently leads CCI’s Embryonal Cancer Therapy and Prevention Group. Marshall was appointed a Member of the Order of Australia in 2014.
Associate Professor Rosemary Sutton
Rosemary Anne Sutton graduated with a Bachelor of Science from the Australian National University then completed a PhD at the University of Sydney in 1979. Sutton joined CCI in 2001 and led the MRD group until her retirement in 2021. During this time, she was responsible for overseeing the diagnostic testing of over 3,000 patients with acute lymphoblastic leukaemia from Australia and New Zealand for 11 different ALL clinical trials.
Dr Toby Trahair
Toby Trahair is a paediatric haematologist, oncologist and haematopoietic stem cell transplant physician. He completed PhD studies in gene therapy at the Children’s Medical Research Institute, at Westmead Hospital in Sydney, prior to completing specialist training in paediatric haematology and oncology. Trahair is a staff specialist at the Kids Cancer Centre and Medical Director of the CCI’s MRD laboratory.
Dr Michelle Henderson
Michelle J Henderson completed a PhD at the University of Sydney (1995), was a research fellow at the Garvan Institute of Medical Research, then joined CCI in 2006, where she is a Senior Scientist. Henderson leads CCI’s MRD laboratory which is also a member of the EuroMRD Consortium (which consists of over 65 laboratories across 25 countries).
Partner
This case study was developed with input from Professor Murray Norris and colleagues and in partnership with CCI.

References
The information and images from which impact case studies are produced may be obtained from a number of sources including our case study partner, NHMRC’s internal records and publicly available materials. Key sources of information consulted for this case study include:
1 Australian Institute of Health and Welfare 2020. Australia’s children. Cat. no. CWS 69. Canberra: AIHW
2 Australian Institute of Health and Welfare 2021. Cancer in Australia 2021. Cancer series no. 133. Cat. no. CAN 144. Canberra: AIHW
3 Erdmann F, Frederiksen LE, Bonaventure A, Mader L, Hasle H, Robison LL, Winther JF. Childhood cancer: survival, treatment modalities, late effects and improvements over time. Cancer epidemiology. 2021 Apr 1;71:101733
4 Cancer Council NSW (2022) About acute lymphoblastic leukaemia (ALL), accessed 26 March 2024. https://www.cancercouncil.com.au/acute-lymphoblastic-leukaemia/about-acute-lymphoblastic-leukaemia-all/
5 Cancer Council NSW, Dramatically improving survival for the most common childhood cancer, accessed 26 March 2024. https://www.cancer.org.au/about-us/how-we-help/research/stories/dramatically-improving-survival-for-the-most-common-childhood-cancer
6 Parker C, Krishnan S, Hamadeh L, Irving JA, Kuiper RP, Révész T, Hoogerbrugge P, Hancock J, Sutton R, Moorman AV, Saha V. Outcomes of patients with childhood B-cell precursor acute lymphoblastic leukaemia with late bone marrow relapses: long-term follow-up of the ALLR3 open-label randomised trial. The Lancet Haematology. 2019 Apr 1;6(4):e204-16
7 Sutton R, Shaw PJ, Venn NC, Law T, Dissanayake A, Kilo T, Haber M, Norris MD, Fraser C, Alvaro F, Revesz T. Persistent MRD before and after allogeneic BMT predicts relapse in children with acute lymphoblastic leukaemia. British Journal of Haematology. 2015 Feb;168(3):395-404
8 Greenwood M, Trahair T, Sutton R, Osborn M, Kwan J, Mapp S, Howman R, Anazodo A, Wylie B, D'Rozario J, Hertzberg M, Irving I, Yeung D, Coyle L, Jager A, Engeler D, Venn N, Frampton C, Wei AH, Bradstock K, Dalla-Pozza L. An MRD-stratified pediatric protocol is as deliverable in adolescents and young adults as in children with ALL. Blood Advances 2021 Dec 28;5(24):5574-5583
9 Application for Medicare Benefits Schedule and other public funding. Application 1703 – Detection of minimal residual disease in patients with acute lymphoblastic leukaemia. The Royal College of Pathologists of Australasia. 2021. Accessed 5 April 2024. http://www.msac.gov.au/internet/msac/publishing.nsf/Content/1703-public