The Australian Government is now in caretaker period. During this time, updates on this website will be published in accordance with the Guidance on Caretaker Conventions, until after the election.
Up until the mid-20th century there was little that clinicians could do to help a patient with renal (kidney) failure: the condition was fatal. By the late 1960s, however, advancements in medical research had made it possible to replace diseased kidneys with healthy ones through transplantation and to keep patients alive with dialysis until donor kidneys became available. NHMRC-funded clinician researchers played key roles in transforming kidney transplantation from an experimental procedure to a world-leading health care service for Australians with renal disease.
Origin
The kidneys are essential organs, maintaining a state of homeostasis (balance) within the body.
A major function of the kidneys is to remove waste products, toxic molecules and excess fluid from the body. Each minute about one litre of blood enters a person’s kidneys through the renal arteries and leaves – cleansed – back into the renal veins. The waste products are filtered from the blood and flow as urine down the ureters and into the bladder. The production of urine involves highly complex steps of excretion and re-absorption.
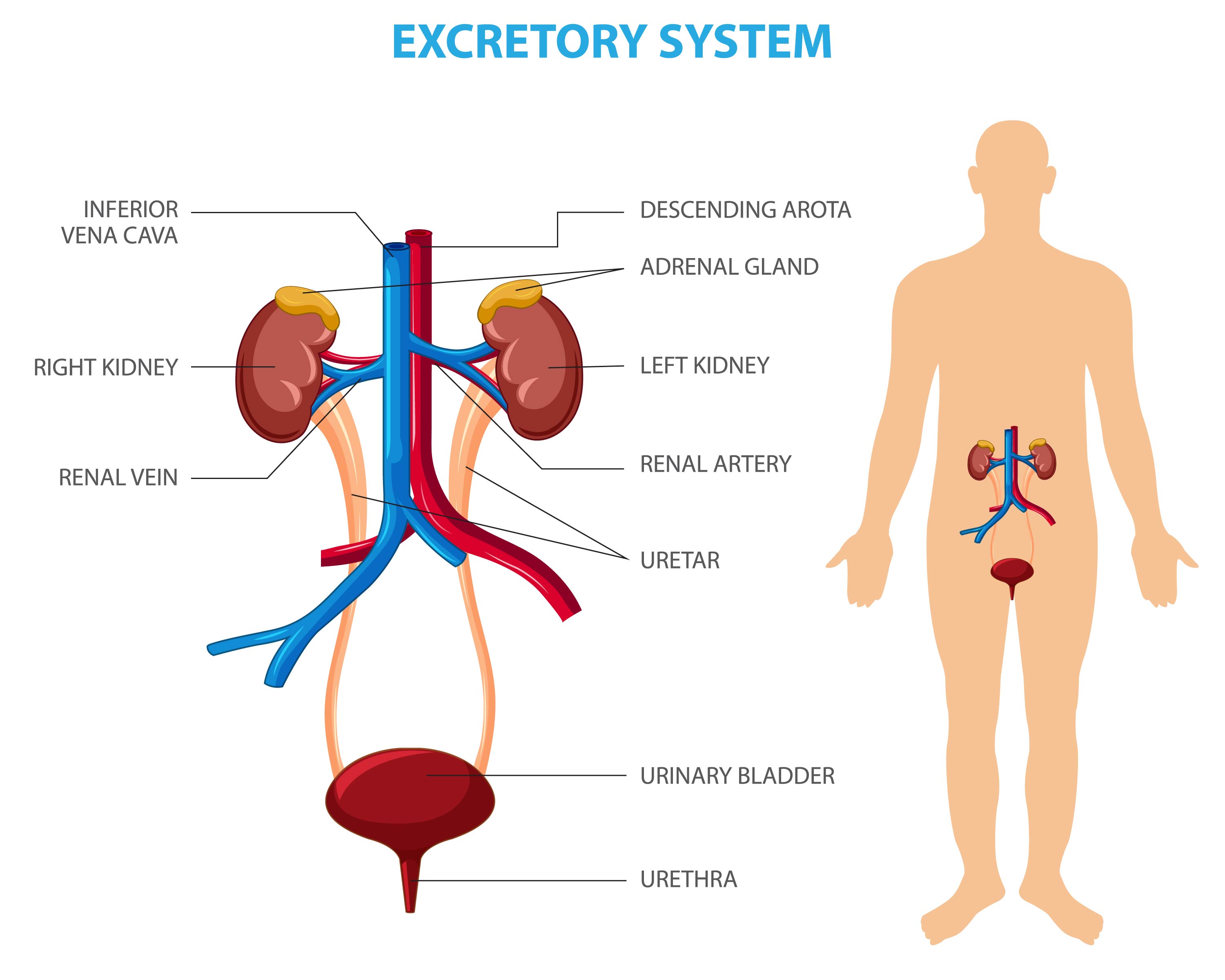
However, the kidneys do much more than this. They also regulate the body's salt, potassium and acid content and they produce hormones that affect the function of other organs. For example, a hormone produced by the kidneys stimulates red blood cell production, while other hormones help regulate blood pressure and control calcium metabolism. In addition, the kidneys produce an active form of vitamin D that promotes strong, healthy bones.
Because kidneys are essential organs, if – as a consequence of kidney disease – a person loses the use of their kidneys and does not have some other mechanism for cleaning their blood – they will die.
However, because of the many functions that kidneys perform, a functioning kidney is a significant improvement over dialysis, a process that allows blood to be filtered by a semi-permeable membrane in an external artificial kidney.
During the late 1950s, an immunosuppression drug (azathioprine) was announced that could, in combination with corticosteroids, enable kidneys to be transplanted without rejection. Because a person’s immune system will reject foreign tissues and organs, immunosuppression drugs are needed to make transplantation possible.
Once it became possible to treat end-stage renal disease there was a rapid increase in activity by kidney specialist clinicians and researchers worldwide. Although azathioprine only became available in Australia in 1964,1 by 1968, renal transplant operations had become an established surgical procedure in major hospitals.2
Clinical practice was, however, racing far ahead of basic knowledge about kidney function and disease, immunology, organ rejection and how azathioprine actually worked.1,3,4 This meant that, when kidney transplantation first began in Australia, mortality and graft loss were high and the rate of long-term success was low – with less than 50% of transplanted kidneys (grafts) surviving three years. Significant morbidity and mortality was also occurring from the side effects of the immunosuppressive drugs and from unsuccessful transplants.1,5,6 Moreover, even when the results of transplantation were successful, clinicians did not fully understand why.7
Improved knowledge was needed, and providing this knowledge would become a focus for NHMRC and for NHMRC-funded clinicians at major hospitals and universities.
Investment
The possibility of successful kidney transplantation had created the need for an entirely new type of capacity within Australian hospitals, so in 1966, NHMRC created a committee to provide recommendations on the rationalisation of facilities for organ transplantation and renal dialysis in Australia.8 The committee’s membership included Priscilla Kincaid-Smith (Royal Melbourne Hospital), Peter Morris (University of Melbourne), John Loewenthal and Ross Sheil (University of Sydney) and James Lawrence (The Queen Elizabeth Hospital in South Australia).7
The committee’s report, adopted by NHMRC’s Council in October 1968,7 described how hospital organ transplant and renal units should be structured and resourced. It noted the primary importance of preventing renal disease in the first instance, the shortage of donor organs, that the larger the donor pool the greater the chance of providing well-matched organs, that close cooperation between hospital authorities was therefore essential, and that additional research was required in a wide range of areas.
It proposed the predominant use of kidneys obtained from deceased donors as opposed to those obtained from living donors.
It recommended that a national register should be established to record patients under treatment by dialysis and undergoing transplantation. The register was to be both a monitoring tool and a research tool. It would enable the rapid identification and evaluation of trends in treatment methods and results, and it would facilitate feedback from the hospital to the laboratory.9
In response to the committee’s report, Council agreed that there was an urgent need for the provision of special funds for the development of major renal units across Australia, and it subsequently established a committee – whose membership included Morris – for the evaluation of research proposals in immunology and organ transplantation.10 NHMRC then increased funding for grants in the areas of renal disease and transplantation surgery.
NHMRC also awarded funding to Loewenthal to develop the national register and form a National Transplantation Registry Sub-Committee. Funding to support the register was also, subsequently, provided by the Australian Kidney Foundation.
In addition, NHMRC formed a committee on renal diseases, chaired by Lawrence, whose work focused on developing Australia’s renal medicine workforce.11
The PDF poster version of this case study includes a graphical time showing NHMRC grants provided and other events described in the case study.
Research
Over time, NHMRC funded many researchers to advance knowledge relevant to kidney transplantation. The work of some of the leaders within major universities and hospitals is described below.
Royal Melbourne Hospital (RMH) and the University of Melbourne (UoM)
A research team including Peter Morris, Alan Ting, John Stocker and John Fabre at the University of Melbourne made a number of contributions to improving the success of kidney transplantation.
Working with Ian McKenzie, they provided the first description of cytotoxic antibodies in humans against pig and sheep lymphocytes. This would be a critical hurdle in any future attempts at xenotransplantation (i.e. transplantation of organs from other animals to humans).
They played an important role in identifying the improved outcomes of patients who had had pre-transplant blood transfusions. They also established, early on, that some patients were likely to develop acute vascular rejection of a graft from a given donor due to an immune-system response that could be identified through a special kind of test (a B-cell crossmatch test). This observation preceded the later development of HLA matching.
HLA stands for Human Leukocyte Antigen complex and refers to a component of the immune system. Because differences in HLA between the kidney donor and recipient were thought to be most important in causing a recipient’s immune system to respond and reject the transplant, a key step in improving transplantation success rates was the ability to match HLAs between donor and recipient, which would in turn allow a lower dose of immunosuppressive drugs and steroids to be used. This was important because long-term immunosuppression increased rates of cancer and susceptibility to infectious disease, while long-term use of steroids could cause cataracts.
A major accomplishment of Morris and his colleagues was the establishment of an HLA Tissue typing Laboratory at Royal Melbourne Hospital.
Developing understanding of the HLA system was an international endeavour and, in 1968, Morris’ laboratory was nominated by the World Health Organization as one of fifteen international tissue typing reference laboratories that would work collaboratively to understand the system.
By 1970, Morris’s team was able to show that a well-matched kidney transplant had approximately double the chance of surviving three years than did a poorly-matched transplant.
Because of the complexity of the HLA system, the best chance of a successful transplant occurs when there is a large group of patients from whom to find the right match for a particular donor. For this reason, Morris’ team and others around Australia and in New Zealand created a combined national waiting-list for patients requiring transplantation. Morris’ group supported this work by leading the development of a jointly agreed methodology and materials for tissue-typing, a national tissue-typing register and a computerised system of recipient and donor pools.12
At the University of Melbourne, and working with Morris, surgeon Vernon Marshall, along with Gordon Clunie and Ian Hardie at the University of Queensland, worked to develop preservation solutions for transplanted kidneys. Such solutions were necessary to diminish injury to the kidney when it was no longer receiving a blood supply and during cold storage, and ultimately to improve graft survival. They developed hyperosmolar citrate solution (also known as Marshall’s solution), whose details were published in 1976.13
University of Sydney
The work of Ross Sheil’s team at the University of Sydney was focused on defining what better and safer immunosuppression looked like, by analysing the way that immunosuppressive agents such as steroids and azathioprine worked.3 They investigated the use of an immunosuppressive treatment called antilymphocyte globulin (ALG) and found that the globulin was safe for use if care was exercised in dosage.
Sheil’s team also undertook research on organ preservation and storage for transplantation.12 They found that when kidneys were removed in optimum circumstances, good kidney function could be expected for up to 10 hours between extraction and implantation, a time adequate for transfer of organs between the most distant Australian and New Zealand cities.14
University of Queensland
As noted above, Clunie, Hardie, Geoffrey Collins and other clinician researchers at the University of Queensland (UQ) investigated how best to preserve kidneys following their removal from the donor and before their insertion into the recipient. The UQ team investigated a variety of fluids for injecting into the kidney during transportation and also developed a series of simple tests to determine how much damage a kidney had undergone before it was preserved. The preservation technique they developed – involving ice storage and ‘machine perfusion’ (continuous injection of a cold fluid into the stored kidney) allowed preservation periods of up to 24 hours with good results.2,1
The solution that they developed (the ‘Collins solution’) was announced in 1969. In a modified form, it became known as EC Solution and was the first such solution developed in the world.15
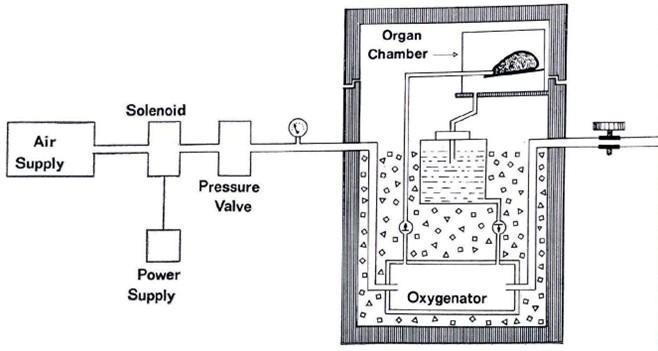
Source: UQ and NHMRC21
The Queen Elizabeth Hospital and University of Adelaide
Clinicians and researchers in Adelaide were kidney transplant pioneers. The first successful renal transplant in Australia was performed in February 1965 at The Queen Elizabeth Hospital in South Australia, by Professor Peter Knight (recipient surgeon) with donor surgeon Dr William (Bill) Proudman AO.
Professor Knight had been recruited by the University of Adelaide from the Peter Brent Brigham Hospital in Boston where he had worked with future Nobel Laureate Dr Joe Murray who had performed the first successful kidney transplant in the world.
Unlike other early kidney transplants, the first Australian kidney transplant was a living unrelated transplant (father-in-law giving to son-in-law), and it was the first to last longer than one year (it ultimately lasted for almost 13 years).
The Queen Elizabeth Hospital continued to lead in transplantation immunosuppression trials through the work of Associate Professor Tim Mathew AM, Professor Graeme Russ and Associate Professor Prof Mohan Rao AM, who introduced laparoscopic donor nephrectomy surgery to Australia in 1996.
ANZDATA
The NHMRC-supported transplant register developed by Loewenthal and Sheil, and a dialysis register developed by Lawrence, combined in 1976 to become the Australian and New Zealand Dialysis and Transplant (ANZDATA) Registry (now funded by the Australian Organ and Tissue Authority and located at the South Australia Health and Medical Research Institute (SAHMRI)).12,16 ANZDATA operations commenced in 1977 with the first data input in October of that year.
The ANZDATA registry (and renal registries in general) were forerunners of Clinical Quality Registries (CQRs). Regular reporting at a national scale of dialysis and kidney transplant activity and close integration with clinical groups underpinned improvement in access to and outcomes of treatment across the country.17,18 CQRs now underpin health service planning, quality improvement and clinical research in many areas.
Translation
Many of the activities listed above have led to important features of contemporary clinical practice in Australia and worldwide.
- The NHMRC committee’s report on rationalization of facilities for organ transplantation and dialysis strongly influenced the future development of kidney transplantation in Australia and led to an integrated national approach based on organ exchange between renal units and the close integration of dialysis and transplantation.19,2,3 Demand for the report was so high that it was printed as a booklet and widely distributed in Australia and overseas.20
- By 1970, a national kidney exchange program had developed between all transplantation units in Australia and New Zealand that still exists today.17
- The Committee’s recommendation that care of end-stage renal failure should be centralised led to a level of cooperation and sharing of kidneys in Australia that other countries had not reached even 20 years later.21
- ANZDATA has recorded data on all patients in Australia who have ever been treated for end-stage renal failure by dialysis or renal transplantation, making it the longest continuous dataset of its type in the world. It has been a central resource for all kidney transplant and dialysis related research since its inception and has enabled Australian researchers to contribute to major international clinical trials related to kidney transplantation.28
- HLA matching has produced major impacts on transplantation success. The Red Cross assisted in setting up a network of tissue-typing laboratories in each major Australian city. 22,23
- EC solution was commonly used clinically for 15 years after its announcement.24 Marshall's solution is one of two major types of fluid still used for the preservation of kidneys.25
- While machine perfusion was superseded by static cold storage (SCC) for 25 years, it is now central to attempts to repair organs pre-transplant and has been found to be superior to SCC.26
- ALG is still used for the suppression of the immune system to prevent renal transplant rejection.
Outcomes and impacts
Kidney transplantation (along with other solid organ transplantation) was one of the greatest medical advances during the second half of the 20th century, enabling significantly prolonged life for patients with kidney disease.
Kidney transplantation in Australia has a long history of success, with world-leading graft and patient survival. Underpinning this success has been a commitment to basic and clinical research in transplantation and immunology, with strong regional and global collaborations. Although conducting <2% of global transplants, Australian sites have participated in most major multinational studies in transplantation immunosuppression.
While between 1963 and 1968, less than 300 kidney transplants in total had been performed in Australia, by the early 1980s, more than 400 transplants were being performed each year.27,28
By the early 1990s, average renal transplant survival rate across Australia rivalled those of the better individual transplant units elsewhere in the world.20,29 As shown in the figure below, contemporary rates of graft survival are higher still.
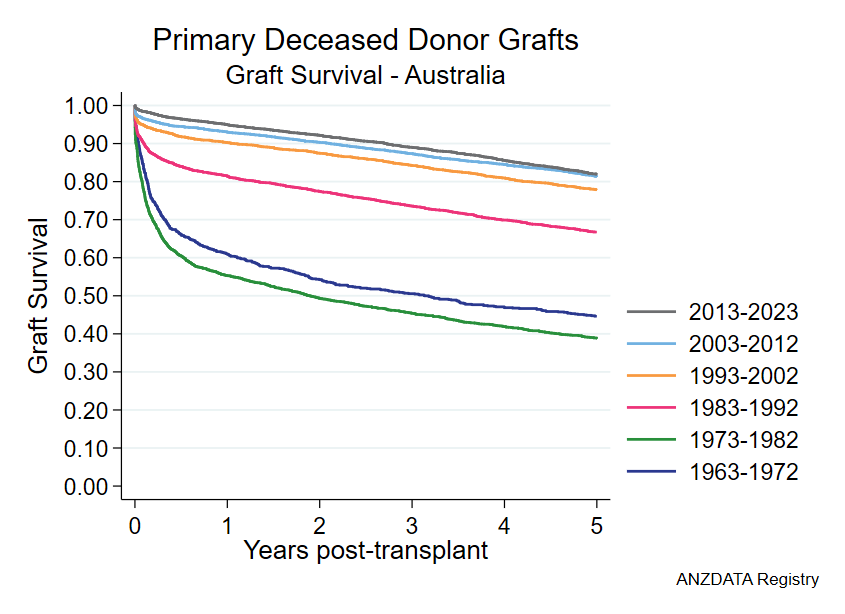
These successes have been a direct consequence of the high level of research engagement by transplant specialists in Australian hospitals. Soon after each therapeutic innovation has been developed, and regardless of where in the world it originated, Australian clinicians have been able to rapidly apply them to their patients.26
Australia’s national kidney transplantation capability remains world-leading.30,* It is based on a model of central coordination and collaboration and underpinned by the ANZDATA registry, which has been critical to the identification of key elements to successful kidney transplantation and assuring appropriate access to and outcomes of transplantation.
In 1983, the US Food and Drug Administration approved a new and significantly improved type of immunosuppression drug (ciclosporin) for clinical use, opening a new era for kidney transplantation. Australia was involved in some of the critical early trials for this drug and has had an ongoing major role internationally in trials of new immunosuppressive agents. Australian researchers Jeremy Chapman AC and Tim Mathew AM (Director of the Renal Unit at The Queen Elizabeth Hospital (1977-2002) and a key developer of ANZDATA) were at the centre of this work.
* Systemic inequality means that Aboriginal and Torres Strait Islander peoples experience a higher burden of disease and lower life expectancy than non-Indigenous Australians. Chronic conditions significantly contribute to this burden, which is further exacerbated by lack of access to interventions such as kidney transplants.
Researchers
Professor Sir John Loewenthal CMG
John Isaacs Loewenthal (1914-1979) studied medicine at the University of Sydney (USyd). He was a resident medical officer then senior resident pathologist at Royal Prince Alfred Hospital (RPAH) (1938-40). He served as a Captain in the Australian Army Medical Corps during the Second World War (1940-1945). During the war he undertook research on the clinical use of penicillin, and for this work, was awarded a Master of Science by the University of Melbourne (UoM) in 1946.
From 1946-1948, Loewenthal worked in the United Kingdom (UK) on a Nuffield travelling fellowship in London at St Bartholomew's Hospital, then was appointed lecturer in surgery at the Victoria University of Manchester. In 1948, he returned to Sydney where he began private practice but also worked as a surgeon at RPAH. In 1956, Loewenthal became Bosch Professor of Surgery at USyd, then Dean of the Faculty of Medicine (1965-71).
Professor Priscilla Kincaid-Smith AC CBE
Priscilla Sheath Kincaid-Smith (1926–2015) was born in South Africa and worked at Baragwanath Hospital in Johannesburg, holding resident positions in Medicine and Surgery and Registrar in Medicine (1951–1953). She was RMH Director of Nephrology (1967–91); Professor of Medicine at the UoM (1975–91); and Physician in Nephrology, Royal Women's Hospital, Melbourne (1976–91).
Kincaid-Smith was President of the Royal Australasian College of Physicians (1986–88), as well as past president of the World Medical Association, and International Society of Nephrology. She was a President of the Royal Australasian College of Physicians (1986-1988) and of the International Society of Nephrology (1972–75). In 1989, Kincaid-Smith was appointed a Companion of the Order of Australia for service to medicine, particularly in the field of nephrology.
Professor Sir Peter Morris AC
Peter John Morris (1934-2022) graduated in medicine from UoM in 1957, completed a residency at St Vincent’s Hospital in Melbourne, then moved to the UK and United States to complete his training. Returning to Australia in 1968, Morris joined the UoM Department of Surgery.
In 1973, he was appointed to the Nuffield Chair of Surgery at the University of Oxford. He held this post for 28 years before being elected as President of The Royal College of Surgeons of England (2001-2004). Morris was awarded a knighthood for his services to medicine in 1996. In 2004, he was made a Companion of the Order of Australia for services to medical science.
Professor Jim Lawrence AO
James (Jim) Roland Lawrence (1930-2018) graduated in medicine at the University of Adelaide (UoA) in 1954 then became Resident Medical Officer at the Royal Adelaide Hospital and the Adelaide Children’s Hospital. Commencing in 1956, he worked as a Lecturer in Pathology and Research Fellow at UoA. From 1958 to 1959 he was a Research Assistant and Fellow at the University of Illinois. In 1961, he moved to Edinburgh Royal Infirmary and then to London University College Medical School.
He returned to Adelaide in 1964 as Director of the new Renal Unit at Queen Elizabeth Hospital. There, in 1965, he was part of the team that performed the first successful kidney transplant in Australia. In 1976, he was appointed Professor of Medicine at USyd and Foundation Chair at the Repatriation General Hospital, Concord, which he remained until 1997. From 1988 to 1991, he was Head of the Department of Medicine at USyd.
Professor Gordon Clunie
Gordon James Aitken Clunie (1932-2016) graduated in medicine at the University of Edinburgh in 1956 then trained in surgery in Edinburgh and Newcastle-Upon-Tyne. He was awarded Surgical Fellowships of the College of Surgeons of Edinburgh (1963) and England (1964) then became Senior Registrar and Lecturer in the Department of Surgery at the Royal Infirmary in Edinburgh.
In 1968, Clunie moved to the UQ Department of Surgery at the Princess Alexandra Hospital in Brisbane as Director of the new Dialysis and Renal Transplant Unit. He was later appointed Professor of Surgery and Deputy Dean in UQ’s Faculty of Medicine. From 1978 to 1995, Clunie was the James Stewart Professor of Surgery at UoM and the Royal Melbourne Hospital. At UoM he was appointed Assistant-, Deputy- and then Dean of the Faculty of Medicine, Dentistry and Health Sciences (1980-1997). He was the Editor and then Editor in Chief of the Australian and New Zealand Journal of Surgery (1983-1996).
Professor Ross Sheil AO
Ainslie Glenister Ross Sheil (1933-) studied medicine at UQ. In 1957, he was awarded a Rhodes scholarship to Balliol College, Oxford University, where he continued his studies in medicine. Sheil was admitted to the Royal College of Surgeons in 1962. After further training in the UK and US, Sheil returned to Australia where he specialised in vascular surgery.
He was appointed Professor of Transplant Surgery at USyd and formed part of the Renal Transplant Group there, which in 1967 embarked on a renal transplant program for NSW. He headed the Transplantation Unit at RPAH and became head of the Australian National Liver Transplant Unit and carried out the first liver transplant in NSW. He was appointed Officer of the Order of Australia in 1992 for services to surgery particularly clinical and research transplantation.
Professor Alan Ting
Alan Ting (1943-2017) undertook an Honours degree in Zoology graduating in 1964 at USyd. He moved to the RMH blood transfusion laboratory then, in 1972, completed his PhD supervised by Peter Morris at the UoM Department of Surgery. From 1973-75 he undertook postdoctoral research in the United States, then in 1975 joined Morris in Oxford as a senior research officer. Following 15 years in Oxford, Ting was Director of the Histocompatibility Laboratory at the California Pacific Medical Centre (1990-1994) then Associate Professor of Pathology and Co-Director of the Histocompatibility Laboratory at Stanford University. He was appointed as President of the American Society of Histocompatibility and Immunogenetics (ASHI) in 1999 and in 2000 became Assistant Director, then later, Director of Research at the United Network for Organ Sharing (UNOS) to Richmond, Virginia.
Professor John Stocker AO
John Wilcox Stocker (1945-) completed his Bachelor of Medical Science at UoM in 1969, and a Bachelor of Medicine/Bachelor of Surgery in 1970. In 1976, he completed his PhD at the Walter and Eliza Hall Institute of Medical Research in Melbourne, then joined the Basel Institute for Immunology in Switzerland. In 1979, Stocker took a position with Hoffmann–La Roche in Basel where he would become Director of Pharmaceutical Research. In 1987, Stocker returned to Australia as the founding Managing Director of the commercial biomedical company, AMRAD Corporation Ltd.
Stocker was Chief Executive Officer of CSIRO (1990-1995), Chief Scientist of Australia (1996-1999), and Chair of the CSIRO Board (2007-2010). He was made an Officer in the Order of Australia in 1999 for services to Australian scientific and technological research.
Professor Vernon Marshall AO
Vernon Charles Marshall was a general surgeon at Footscray Hospital (1963–1991) and held the post of Professor of Surgery at Prince Henry’s Hospital while he worked at Footscray. Marshall was Head of the Department of Surgery at Monash University from 1975 to 1996. He was then Editor and Head of the Australian Medical Council’s Examination Committee for overseas doctors. Marshall authored a number of books on surgery and surgical education, and co-authored the undergraduate surgical textbook, Clinical Problems in General Surgery. In 2018, Professor Marshall was appointed an Officer of the Order of Australia for his service to medicine.
Partners
This case study was developed with input from Professor Jeremy Chapman AC, Professor Stephen McDonald, Professor Toby Coates AO and in partnership with ANZDATA.

References
The information and images from which impact case studies are produced may be obtained from a number of sources including our case study partner, NHMRC’s internal records and publicly available materials. Key sources of information consulted for this case study include:
1 Kincaid-Smith P, Lawrence JR, Sands J, Morris PJ and Sheil AGR. Australian national dialysis and renal transplantation survey. First report by a subcommittee. Lancet 1: 744, 1970
2 Director-General of Health. Annual report by the Director-General of Health for the year 1967-68. Commonwealth Government, Canberra, 1969
3 National Health and Medical Research Council. National Health and Medical Research Council Thirty-First Annual Report for 1968. Commonwealth Government, Canberra, 1969
4 National Health and Medical Research Council. National Health and Medical Research Council Thirtieth Annual Report for year 1967. Commonwealth Government, Canberra, 1969
5 National Health and Medical Research Council. National Health and Medical Research Council Report for year 1970. Commonwealth Government, Canberra, 1972
6 National Health and Medical Research Council. Medical Research 1965. Commonwealth of Australia, Canberra, 1966
7 National Health and Medical Research Council. National Health and Medical Research Council Sixty-seventh Session, Canberra, 24-25 October 1968, Volume 1. Commonwealth of Australia, Canberra, 1969
8 National Health and Medical Research Council. National Health and Medical Research Council Sixty-third Session, Canberra, 4 November 1966. Commonwealth of Australia, Canberra, 1966
9 Kincaid-Smith P, Lawrence JR, Sands J, Morris PJ and Sheil AGR. Australian national dialysis and renal transplantation survey. First report by a subcommittee. Lancet 1: 744, 1970
10 National Health and Medical Research Council. National Health and Medical Research Council Sixty-eighth Session, Brisbane, 15-16 May 1969, Volume 1. Commonwealth of Australia, Canberra, 1969
11 National Health and Medical Research Council. National Health and Medical Research Council report of the seventy-ninth session, Canberra, November 1974. Australian Government, Canberra, 1975
12 National Health and Medical Research Council. National Health and Medical Research Council Seventy-third Session, Canberra, October 1971. Australian Government, Canberra, 1972
13 Ross H, Marshall VC, Escott ML. 72-hr canine kidney preservation without continuous perfusion. Transplantation. 1976;21(6):498–501
14 Sheil AG, Stewart JH, Johnson JR, May J, Storey BG, Rogers JH, Charlesworth JA, Hurley BP, Tiller D, Mahony JF, Bashir H. Cadaveric donor renal transplantation. Medical Journal of Australia. 1972 Jan;1(5):205-9
15 Chen Y, Shi J, Xia TC, Xu R, He X, Xia Y. Preservation Solutions for Kidney Transplantation: History, Advances and Mechanisms. Cell Transplant. 2019 Dec;28(12):1472-1489
16 Disney A. Renal transplantation in Australia and New Zealand, 1963-1982: report from the ANZ Data Registry. Transplantation Proceedings 1984 Aug 1 (Vol. 16, No. 4, pp. 973-978)
17 Disney A. Renal transplantation in Australia and New Zealand, 1963-1982: report from the ANZ Data Registry. Transplant Proc 1984;16(4):973-8
18 Sheil AG, Flavel S, Disney AP, Mathew TH. Cancer development in patients progressing to dialysis and renal transplantation. Transplant Proc 1985;17(2):1685-8
19 George CR. Caring for kidneys in the antipodes: how Australia and New Zealand have addressed the challenge of end-stage renal failure. American journal of kidney diseases. 2009 Mar 1;53(3):536-45
20 Director-General of Health. Annual report by the Director-General of Health for the year 1968-69. Commonwealth Government, Canberra, 1969
21 Mandel DT, Chapman JR. Transplantation in Australia—50 years of progress. Medical journal of Australia. 1992 Jul;157(1):46-50
22 Bashir HV, Subcommittee A. Cadaver renal transplantation and HLA matching in Australia from 1971 to 1980: A report of the Australian and New Zealand Combined Dialysis and Transplant Registry. Transplantation. 1982 Oct 1;34(4):183-9
23 Lawrence JR. The evolution of the end-stage renal disease program in Australia. Renal failure. 1994 Jan 1;16(1):133-46
24 Chen Y, Shi J, Xia TC, Xu R, He X, Xia Y. Preservation Solutions for Kidney Transplantation: History, Advances and Mechanisms. Cell Transplant. 2019 Dec;28(12):1472-1489
25 O'Callaghan JM, Knight SR, Morgan RD, Morris PJ. A National Registry Analysis of Kidney Allografts Preserved With Marshall's Solution in the United Kingdom. Transplantation. 2016 Nov;100(11):2447-2452
26 Tingle SJ, Figueiredo RS, Moir JA, Goodfellow M, Talbot D, Wilson CH. Machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database Syst Rev. 2019 Mar 15;3(3)
27 Sheil AGR, Lawrence JR, Morris PJ, Kincaid-Smith P and Sands J. Australian National Renal Transplantation Survey: Third report by a subcommittee. Medical Journal of Australia 1974, 2: 656-660
28 Kincaid-Smith P, Lawrence JR, Sands J, Morris PJ and Sheil AGR. Australian national dialysis and renal transplantation survey. First report by a subcommittee. Lancet 1: 744, 1970
29 Disney AP. Progress in renal transplantation in Australia during 1975 to 1988. Clinical transplants. 1989 Jan 1:165-73
30 Wyld MLR, Wyburn KR, Chadban SJ. Global Perspective on Kidney Transplantation: Australia. Kidney360. 2021 Aug 5;2(10):1641-1644.