Australia is home to many viruses – called ‘arboviruses’ – that can, or could, infect humans. Some arboviruses cause seasonal illness, others cause epidemics and some can even cause death. During the second half of the 20th century, NHMRC-funded researchers at the Queensland Institute of Medical Research (now QIMR Berghofer) made major contributions to our understanding of arboviruses, enabling clinicians to quickly identify infections in patients and public health authorities to better manage the threats that the viruses pose to health. Research on diseases caused by viruses, bacteria and parasites continues at QIMR Berghofer.
Origin
In 1928, there were two reports of an ‘unusual epidemic’ in Narrandera and Hay in New South Wales. In Hay, patients exhibited symptoms such as skin eruption and painful swelling of the joints.1 Fifteen years later, during the Second World War, several outbreaks of arthralgia (joint pain) and arthritis were described in the Northern Territory, Queensland and the Schouten Islands off the northern coast of Papua New Guinea.2 This syndrome came to be called epidemic polyarthritis but its causes were not clear.
One tropical disease that was known to be present in Australia was dengue. Four large dengue epidemics swept through Queensland and New South Wales between 1897 and 1926. They were responsible for a reported 733 deaths and affected up to 90% of the urban population in each outbreak.3
Arboviruses
Dengue viruses are carried by mosquitoes, which belong to the phylum of animals known as arthropods. This phylum includes insects such as flies, midges, sandflies, blackflies, fleas and lice, and arachnids such as ticks and mites.
Organisms that feed on blood can act as ‘disease vectors’, meaning that they can transmit blood-borne viruses or other pathogens and parasites from one animal to another, potentially resulting in disease epidemics. Viruses spread by insects and arachnids are called arboviruses (arthropod-borne viruses) and their associated diseases are known as ‘vector-borne diseases’.
In 1947, faced with the challenge of understanding and responding to both known and unknown tropical diseases, the Queensland Government established QIMR, with its laboratory located in disused US Army accommodation huts in Victoria Park, Herston.4 Arboviral research was a focus for QIMR from its inception.

Arbovirus transmission cycle
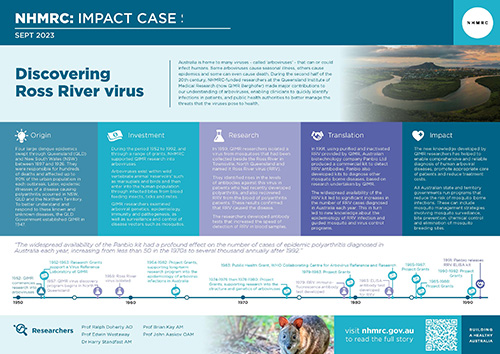
The figure above provides an example of an arbovirus transmission cycle. Ross River virus is maintained in a wild vertebrate animal ‘reservoir’ (often marsupials and birds) but regularly ‘spills over’ into the human population via the infected bites of a large number of mosquito species. Notably, there are some viruses, such as dengue, that don’t have a wild animal host. Dengue is transmitted between viraemic humans (those with virus present in their blood) and susceptible humans by just two mosquito species.
Investment
The Federal Health Council (NHMRC’s precursor) considered health issues related to mosquitos for the first time in 1927, when it noted Australia’s international obligations associated with hygiene at ports. Port hygiene measures were intended to prevent ships from spreading disease through infestations of mosquitoes, rats, lice and contaminated food and drinking water. During the Second World War, NHMRC’s Council began recommending a variety of public health measures related to mosquito control and later formed a sub-committee on the control of Aedes aegypti, the principal mosquito that spreads dengue.
Starting in 1952, NHMRC-funded researchers at QIMR, including Ralph Doherty, Edwin Westaway, Harry Standfast, Brian Kay, John Aaskov, Barry Gorman, Peter Walker and John Carley engaged in a long-term program of research to improve knowledge, prevention and treatment of tropical diseases in general and arboviral diseases in particular. During the period 1952 to 1992, NHMRC supported QIMR’s research in the following areas:
- Virus Reference Laboratory (1952-1963)
- Epidemiology of arthropod-borne and other virus infections in Australia (1964-1982)
- Structure and genetics of arboviruses (1974-1976, 1978-1980)
- An examination of immunity to Ross River virus infection (1979-1983)
- World Health Organisation (WHO) Collaborating Centre for Arbovirus Reference and Research (1983)
- Pathogenesis of Ross River virus infection in man and other animals (1985-1987)
- Prediction and control of medically-important arthropod-borne disease (1985-1986)
- Contemporary techniques for diagnosis of Australian flaviviruses (1987-1988)
- Improved surveillance and ecology of the dengue vector Aedes aegypti (1990-1992).
As described below, research at QIMR has continued in these areas up to the present time, however this case study is focused on the outcomes arising from NHMRC-funding provided during the period 1952-1992.
The PDF poster version of this case study includes a graphical timeline showing NHMRC grants provided and other events described in the case study.
Research
In 1957, Ralph Doherty began a program of virus discovery as part of QIMR’s mission to investigate ‘fevers of North Queensland’. This program was initially based at the QIMR field station in Innisfail and later at Mitchell River Mission/Kowanyama. In 1959, Doherty and colleagues isolated a virus from Aedes vigilax mosquitoes that had been collected beside the Ross River in Townsville, North Queensland and named it Ross River virus (RRV). They then identified rises in the levels of antibodies against this virus in patients who had recently developed epidemic polyarthritis, indicating their symptoms were due to infection with RRV or a virus very closely related to it.5 Subsequent recovery of RRV from the blood of epidemic polyarthritis patients by scientists at QIMR confirmed that RRV caused this disease.
During 1979 and 1980, an epidemic of polyarthritis occurred in Fiji, New Caledonia, Samoa, and the Cook Islands, the first known outbreak outside Australia.6 During this epidemic, QIMR researchers and their Fijian colleagues provided evidence that RRV crossed the placenta in pregnant women, albeit without any apparent effect on the foetus.7
The early serological (blood serum) tests used to diagnose epidemic polyarthritis were slow and cumbersome and often unable to provide a definitive diagnosis. However, in 1979, QIMR researchers developed an indirect immunofluorescence assay to detect anti-RRV IgM antibodies, the initial antibody produced by the immune system in response to a foreign pathogen. These antibodies are difficult to detect as they are only present in blood for a few weeks after infection.8
The new assay enabled doctors to make a diagnosis of epidemic polyarthritis with a single blood sample rather than the two samples collected a week apart that were required with the earlier method of testing. In 1983, QIMR scientists developed an Enzyme Linked Immunosorbent Assay (ELISA) to detect anti-RRV IgM antibodies, which enabled blood samples from hundreds of patients to be tested in a single day.9
Translation
In 1991, Australian biotechnology company Panbio Ltd (now part of Abbott Laboratories) produced a commercial ELISA kit to detect anti-RRV antibodies, using purified and inactivated RRV provided by QIMR.
Panbio also developed kits to diagnose other arboviral diseases like dengue, Barmah Forest virus disease (which manifests similarly to the epidemic polyarthritis caused by RRV) and Japanese encephalitis. The development of these latter diagnostic ELISA kits was also based on research undertaken by QIMR.
The widespread availability of the Panbio ELISA kit had a profound effect on the number of cases of epidemic polyarthritis diagnosed in Australia each year, increasing from less than 50 in the 1970s to several thousand annually after 1992.10 This led to new knowledge about the epidemiology of RRV infection and provided metrics with which mosquito and arbovirus control programs could measure the outcomes of their activities. Panbio ELISA kits have also been used to demonstrate that significant numbers of residents of some Pacific Island countries continue to be infected with RRV and that the infections are not being diagnosed.11
The number, location and times of infections of epidemic polyarthritis revealed by comprehensive testing of suspected patients by Australian pathology laboratories significantly added to knowledge. It demonstrated that RRV has both sylvatic (jungle/bush) and urban cycles of transmission, that both involve zoonotic spillover (transmission from native and domestic animal reservoirs to humans) and that humans are being infected with RRV all year round.

Outcomes and impacts
The most significant outcomes from the studies at QIMR between 1950 and 2000 have been the identification of ‘new’ arboviruses able to cause human disease, development of the capacity to diagnose and prevent these diseases, and the contribution of rapid diagnostics to the process of tracking notifiable arboviral infections nationally.10
During those years, the team at QIMR isolated more than 30 different arboviruses from arthropods collected in Australia.12 Many of these were the first reports of these viruses in Australia and most were new to science. Several have been shown to be the cause of previously unrecognised disease in humans (e.g. RRV and Kunjin virus).13
QIMR Berghofer’s Mosquito Control Laboratory continues to work on surveillance tools, transmission pathways, public health risks and disease mitigation. The ‘Doherty collection’ of viruses, augmented by John Aaskov and the WHO reference centre, continues to serve as a key resource for research on arboviruses and arthritic disease at QIMR Berghofer, curated by its Inflammation Biology Laboratory.
Comprehensive and reliable diagnosis of human arboviral diseases helps promote appropriate care of patients and reduces the costs associated with the inappropriate or unnecessary treatment of undiagnosed disease.
In 2022, there were almost three thousand cases of RRV infection reported nationally.10 Infections with RRV and other vector-borne pathogens (e.g. Barmah Forest virus, chikungunya virus, dengue virus, Japanese encephalitis virus, malaria parasites, flavivirus, Murray Valley encephalitis virus and West Nile / Kunjin virus) are recognised as important public health problems across Australia and clinicians are required to notify government health authorities when the disease is identified.10
Up to 12 thousand vector borne disease notifications have been recorded annually over the past decade. Accurate measurement of numbers allows government to fund preventive measures in accordance with the financial, social and public health risks posed by each virus. It also allows the outcome of any interventions to be quantified.
All Australian state and territory governments publish information aimed at assisting the public to reduce their chances of being infected by arboviruses. The governments of South Australia and Western Australia run the Fight the Bite public awareness campaign, which includes online videos and other resources aimed at influencing public behaviour towards infection prevention.
A number of Australian state governments implement mosquito management strategies. In the Northern Territory, the City of Darwin has run an annual mosquito control program since 1983 aimed at eliminating mosquito breeding sites. In Victoria, a state-wide Arbovirus Disease Control Program includes mosquito surveillance, chemical control, bite prevention and elimination of breeding sites. In Queensland, the Mosquito and Arbovirus Research Committee, initiated through a collaboration between QIMR Berghofer and local and state government, helps direct and prioritise operational research relevant to mosquito and arbovirus surveillance and control.
Arboviral diseases are an increasing problem in Australia and internationally. In March 2022, the WHO launched the Global Arbovirus Initiative, noting the increasing frequency and magnitude of outbreaks of arbovirus disease as a consequence of climate change and other factors. Prevention of such outbreaks is a challenge. Notably, there is currently no approved vaccine against RRV. However, based upon QIMR’s work, researchers at the Queensland University of Technology (QUT) have developed an RRV vaccine that has completed phase 3 clinical trials and that could form the basis for an approved vaccine in the future.
Researchers
Professor Ralph Doherty AO
Ralph Leonard Doherty (d.2016) completed his medical studies and internship at The University of Queensland (UQ) in 1950. In 1956, as a QIMR Senior Research Fellow, and supported by a Rotary Foundation scholarship, Doherty obtained a Master of Public Health (Microbiology) from the Harvard School of Public Health. Doherty was QIMR Director (1966-1978) then became Dean of the Faculty of Medicine at UQ, eventually becoming Pro-Vice Chancellor. In this role, Doherty chaired an Australian Government inquiry into the education of medical students in Australia (1987-1988). In 1989, Doherty was appointed an Officer of the Order of Australia.
Professor Edwin Westaway
Edwin George Westaway (1924-2010) was a Flying Officer in the Royal Australian Air Force (1943-1947) then held various administrative positions with the Queensland Government Department of Health (1948-1960). Westaway completed a Bachelor of Science (1960) and a PhD (1965) at UQ, then became a virologist at QIMR (1961-1968). He held various positions at Monash University, becoming Professor in 1985.
Dr Harry Standfast AM
Harry Andrew Standfast (1930-2012) was, in 1949, a cadet in the Queensland Government Department of Health laboratory, then completed a Bachelor of Science at UQ. Standfast served as a malaria control officer in Papua New Guinea and the Solomon Islands (1955-1962). In 1962, he joined QIMR to work on surveys of arboviruses and their vectors. Standfast worked at CSIRO (1970-1990) then undertook consultancy work on local government mosquito control programs in South East Queensland and livestock and dengue issues in the South Pacific. Standfast was appointed a Member of the Order of Australia in 1998.
Professor Brian Kay AM
Brian Herbert Kay (1944-2017) commenced work at QIMR in 1963 as a cadet, then completed a Bachelor of Science (1970) and a PhD (1978) at UQ. He headed QIMR’s Entomology Laboratory (later called the Mosquito Control Laboratory) from 1970 and later became part of QIMR’s senior executive team. Kay retired in 2014 after 51 years at QIMR. Kay was appointed a Member of the Order of Australia in 2005.
Professor John Aaskov OAM
John Gregory Aaskov undertook a Bachelor of Science at UQ, a PhD at the University of Leeds in the UK and a Fellowship from the Royal College of Pathologists. He commenced work at QIMR in 1976 as a post-doctoral scientist and in 1986 moved to QUT, becoming Professor of Virology and Immunology before retiring in 2019. He was Director of a WHO Collaborating Centre for Arbovirus Reference and Research. Aaskov spent almost 20 years as a Reservist in the Australian Army, the last seventeen as a Major in the Royal Australian Army Medical Corps and head of the arbovirus research laboratory in the Australian Defence Force Institute of Infectious Diseases. Aaskov was awarded a Medal of the Order of Australia in 2015.
References
The information and images from which impact case studies are produced may be obtained from a number of sources including our case study partner, NHMRC’s internal records and publicly available materials. Key sources of information consulted for this case study include:
1 Nimmo JR. 1928. An unusual epidemic. Med. J. Aust. 1:549-550
2 Dowling PG. 1946. Epidemic polyarthritis. Med. J. Aust. 1:245-246
3 Lumley GF and Taylor FH. 1943. Dengue. Service Publication No. 3. School of Public Health and Tropical Medicine, Commonwealth of Australia
4 The Queensland Institute of Medical Research. First Annual Report. Year ended 30th June, 1946
5 Doherty RL, Whitehead RH, Gorman BM and O’Gower AK. 1963. The isolation of a third group A arbovirus in Australia, with preliminary observations on its relationship to epidemic polyarthritis. Aust. J. Sci. 26:183-184
6 Aaskov JG, Mataika JU, Lawrence GW, Rabukawaqa V, Tucker MM, Miles JA and Dalglish DA. 1981. An epidemic of Ross River virus infection in Fiji, 1979. Am. J. Trop. Med. Hyg. 30:1053-1059
7 Aaskov JG, Nair K, Lawrence GW, Dalglish DA and Tucker M. 1981. Evidence for transplacental transmission of Ross River virus in humans. Med J Aust. 2:20-21
8 Aaskov JG and Davies CEA. 1979. An immunofluorescence assay for human antibodies to Ross River virus. J. Immunol. Methods. 25:37-41
9 Oseni RA, Donaldson MD, Dalglish DA and Aaskov JG. 1983. Detection by ELISA of IgM antibodies to Ross River virus in serum from patients with suspected epidemic polyarthritis. Bull. World Health Organ. 61:703-8
10 National Notifiable Disease Surveillance Scheme. Australian Department of Health
11 Aubry M, Finke J, Teissier A, Roche C, Broult J, Paulous S, Despres P, Cao-Lormeau VM and Musso D. Silent circulation of Ross River virus in French Polynesia. International Journal of Infectious Diseases. 2015 Aug 1; 37:19-24.
12 Vasilakis N, Tesh RB, Popov VL, Widen SG, Wood TG, Forrester NL, Gonzalez JP, Saluzzo JF, Alkhovsky S, Lam SK, Mackenzie JS and Walker PJ. 2019. Exploiting the Legacy of the Arbovirus Hunters. Viruses. 23; 11(5):471.
13 Doherty RL. 1974. Arthropod-borne viruses in Australia and their relation to infection and disease. Prog. Med. Virol. 17:136-192.
Supplementary information relevant to this case study is available in:
- Harley D, Sleigh A and Ritchie S. 2001. Ross River virus transmission, infection, and disease: a cross-disciplinary review. Clin. Microbiol. Rev. 14:909-932.
- Russell RC. 2002. Ross River virus: ecology and distribution. Annu. Rev. Entomol. 47:1-31.
Partner
This case study was prepared with input from Professor John Aaskov and Associate Professor Gregor Devine, and in partnership with QIMR Berghofer.
