Glaucoma is the leading cause of irreversible blindness worldwide, with an estimated 80 million people affected1 including more than 200,000 Australians.2 Over two decades, NHMRC-funded researchers at the Lions Eye Institute (LEI) and the University of Western Australia (UWA) developed a new approach that has revolutionised glaucoma treatment, leading to safer surgery and improved vision outcomes. With later support from an international industry team, this new glaucoma surgery is now in use worldwide.
Origin
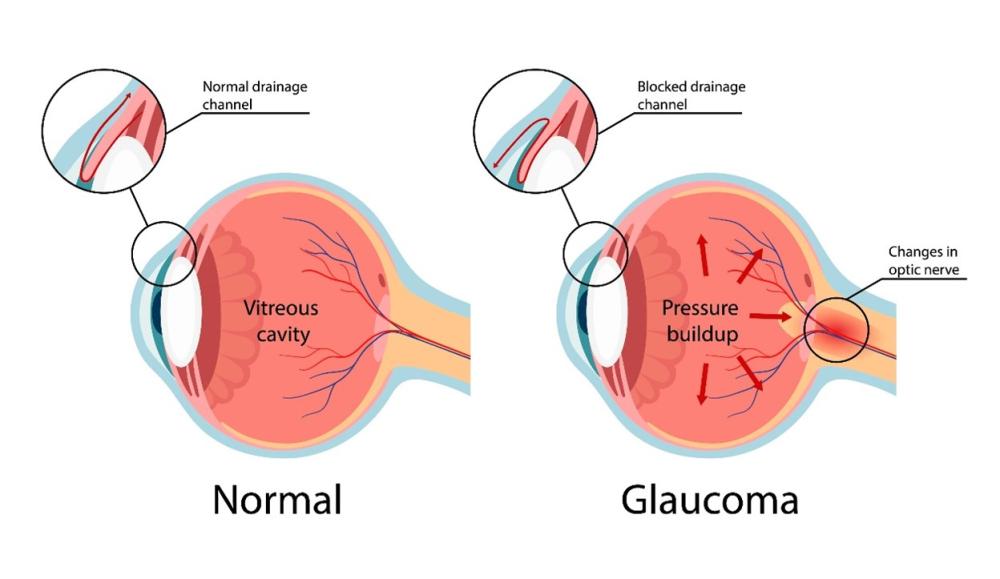
Glaucoma is an eye disease involving optic nerve damage that causes vision-loss and irreversible blindness. It has been known since ancient times. The word glaucoma comes from an ancient Greek word meaning 'blue-green, gray' as this is a common appearance of diseased eyes.3
The front part of the eye contains a transparent, water-like fluid similar to blood plasma that helps to maintain its shape and provide it with nutrients. This fluid enters the eye above the lens, then flows through the iris and exits through the trabecular meshwork, a spongy tissue located near the edge of the cornea, which is the transparent layer covering the front of the eye.
Glaucoma occurs when fluid drainage through the trabecular meshwork is impeded and fluid pressure inside the eye (called intraocular pressure or IOP) increases, leading to damage to the optic nerve, continuous deterioration of vision and eventual blindness.
Effective treatment options for glaucoma include prescription of eye drops, laser treatment and surgery. The goal of these treatments is to decrease IOP. Surgery (called trabeculectomy or TRAB) is performed only when medication and other treatments have not been sufficiently effective.
TRAB is a surgical procedure to bypass the trabecular meshwork by cutting an open channel into the white of the eye. This drains the eye’s fluid into the soft tissue around the eye called the conjunctiva.
Although TRAB has been improved over several decades, this approach is far from ideal. It can cause scarring of the conjunctiva, there is usually a slow recovery of vision after surgery and there can be major complications including leaking of fluid from the surgery site, too much drainage leading to low pressure that causes blurry vision, bleeding inside of the eye, eye infections and cataracts. There are also high rates of failure: the success rate is only about 60% at five years post-surgery.
Professor Dao-Yi Yu was still in training as an ophthalmologist when he published research on how to estimate the aqueous outflow resistance following glaucoma filtration surgery, and over time he became deeply familiar with this and other treatment options for glaucoma. He also became increasingly aware of their limitations and, by the time he arrived at the LEI/UWA in 1985, he was convinced that a better approach was possible.
Investment
NHMRC provided long-term funding for UWA/LEI researchers to support research and skills development relevant to creating a new and improved treatment approach for glaucoma: the gel stent. Funded researchers included Dao-Yi Yu and his team members Bill Morgan, Stephen Cringle, Er-Ning Su, Dean Darcey, Ian Constable and Paula Yu. Successive NHMRC project and program grants, as well as an Australia-China International linkage grant, were held by Professor Yu.
Other sources of funding to support the development of the gel stent and associated technologies included US$100 million venture capital funding to perform clinical trials and commercialise the technology and significant seed funding from the McCusker Charitable Foundation.
The PDF poster version of this case study includes a graphical time showing NHMRC grants provided and other events described in the case study.
Research
Upon his arrival at LEI/UWA, Yu began investigating how to develop the optimal glaucoma treatment. This would need to be a device and surgery that would cause minimal scarring damage to the eye yet provide long-term fluid drainage to the surrounding tissue. The approach used would also need to minimize post-operative complications and speed recovery.
Yu recognised that what was needed was an artificial replacement for the dysfunctional trabecular meshwork. This would be a ‘stent’: a small passage created in the eye allowing fluid to drain away naturally. However, in order for this approach to be widely used a number of challenges would first have to be overcome. This led Yu and the UWA/LEI team to develop multiple elements of a gelatine stent surgery system.
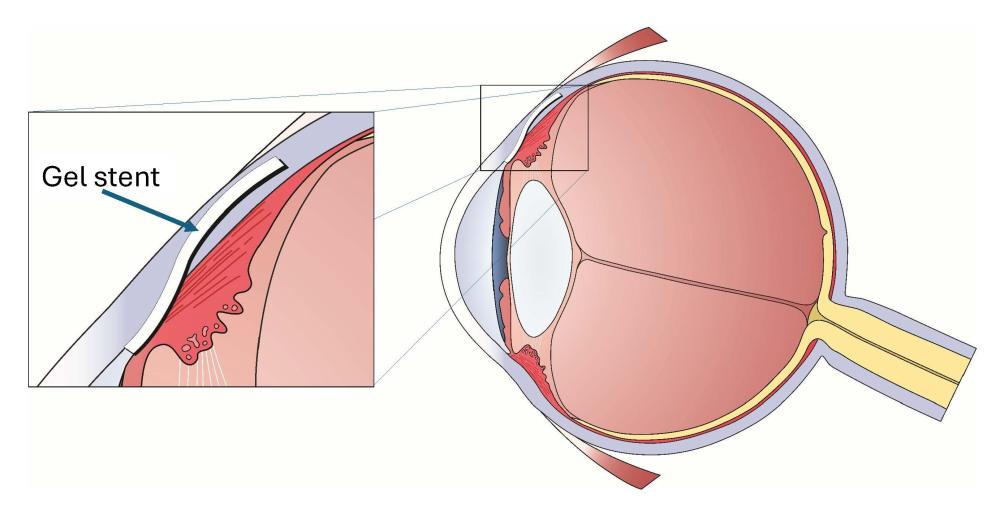
The team developed a tiny, nearly transparent tube to create the stent. The tube is thinner than a human hair, 6mm long (about the length of an eyelash) and is made from cross-linked gelatine, a bio-compatible material.

Developing the tube was a complex process because it had to possess a number of features, including the correct inner and outer diameters and total length, and biocompatibility with the surrounding tissue so as to induce minimal inflammation within the eye.
It also needed to be semi-rigid during the implantation process, to allow quick, safe and accurate implantation, but to be flexible after implantation to conform to the eye shape once implanted and to move with the eye and any deformation force applied to the eye. Such forces occur each time a person blinks and are thought to be the cause of chronic inflammatory reactions found adjacent to plastics and other materials commonly used in implants.
Finally, the tube had to swell after implantation so that it locked into position to prevent any unwanted movement.
A tube with these characteristics did not previously exist. The team made thousands of prototype implants and ran more than 400 tests before finding the right combination of materials.
Developing the tube was only the first step. In order to ensure that it could be safely introduced and correctly placed, the team developed a robotic manipulator and introducer system that would enable precise implantation, including with respect to location, depth and timing.
Each gel stent is provided to the ophthalmologist surgeon within a special-purpose, sterile and disposable needle implanter. The surgery involves implanting the tube in the space between the eye’s anterior chamber and the conjunctiva, the tissue covering the white part of the eye.
Translation
The translation component of the research program to develop the gel stent commenced in 1997 when Yu filed applications for Australian patents for a Biological microfistula tube and implantation method and apparatus and A system for ocular ultramicrosurgery. Extensive experimentation over many years demonstrated the potential for improved surgical outcomes in glaucoma therapy.
For the new glaucoma surgery to become available for treatment worldwide, the technology would need to be commercialised. Yu and his team worked with a US-based medical technology incubator, The Innovation Factory, to raise venture capital, undertook technology transfer activities, assessed local and international clinical trials, obtaining regulatory approvals and attracted interest from large-scale medical device manufacturers.
The new approach to glaucoma surgery includes a bioengineered implant (gel stent), a special one-needle implanter and a procedure (referred to as ab interno) which involves implanting the stent from inside the eye.
The new approach was patented in the United States by LEI in 2003 and the patents were licensed to Aquesys, a US-based start-up in 2006. Yu helped Aquesys raise funding (total amount of US$101.5 million) to underwrite further product development and international clinical trials. Aquesys was later purchased for US$300 million plus future milestone payments by Allergan (now part of AbbVie), which now markets the technology under the commercial name XEN® Gel Stent.
In 2009, and with the assistance of Morgan, Yu performed the first clinical implantations in Australia, then guided the technical development program and multi-centre international clinical trials through to CE Mark approval in Europe in 2015, United States Food and Drug Administration approval in 2016, and Therapeutic Goods Administration approval in Australia in 2017.
The XEN® Gel Stent received approval from Australia’s Medical Services Advisory Committee (MSAC) in August 2017 and, from 1 May 2020, the XEN Gel Stent was listed as a new Medicare Benefits Schedule item. MSAC’s approval was informed by consultation with the Royal Australian and New Zealand College of Ophthalmologists (RANZCO) and the Australian Society of Ophthalmologists.
Outcomes and impacts
Development of the gel stent has revolutionised treatment for glaucoma as it is superior to TRAB in many ways.
Compared to TRAB, surgery is very fast and simple: the gel stents take only 30 minutes to implant, under a local anaesthetic, and implantation involves only a 1.5mm incision into the clear part of the eye, which does not need stitches. There is no conjunctival incision, with the only incision being directly into the fluid compartment of the eye. This greatly reduces the amount of scarring and also permits any subsequent surgeries to be performed in the same location, if necessary, a feature which is not available with other techniques.
Also compared to TRAB, patients recover faster with, generally, less blurred vision, and then have less need of pressure-lowering medication with its attendant side effects. Because the gel stent approach is safer than TRAB it may also be performed earlier in the course of the disease, leading to improved outcomes. Furthermore, the gel stent’s effectiveness at reducing the eye’s pressure is generally better than medication and rivals TRAB.
The implantation of the XEN® Gel Stent has become one of the most common glaucoma surgeries performed globally.4 The stent has now been implanted into over 200,000 patients worldwide (including approximately 3,000 in Australia).5 It is globally recognised as one of the safest and most effective treatments for glaucoma. 6, 7, 8, 9, 10, 11 Published data demonstrates effective drainage and pressure reduction for more than seven years.
The technology has generated a large amount of new clinical research around the world: a recently published review documents the results of almost 200 clinical trials conducted in dozens of countries.4
Numerous publications and surgeons have confirmed satisfaction with the effectiveness and safety of this invention. The incidence of vision threatening complications was low at <1%.4
The team also discovered the critical role of conjunctival lymphatic drainage in the outcomes of glaucoma surgery12 and collaborated with a team of engineers to develop a novel non-invasive and label free conjunctival lymphatic imaging system for clinical use.
Researchers
Professor Dao-Yi Yu AM
Dao-Yi Yu is a clinician scientist. He has a medical doctor degree and PhD degree and worked in the Illinois University at Chicago as a Research fellow. Currently he is a tenured Professor at UWA based at LEI. He is an adjunct Professor of Dalhousie University, Canada and was adjunct Professor of Fudan University, China. Yu is Director of the Lions Eye Institute’s Physiology and Pharmacology group. Yu was appointed a Member of the Order of Australia in 2019, for significant services to ophthalmology and to education.
Professor Bill Morgan
William (Bill) Morgan graduated in medicine from UWA and was later awarded a PhD from UWA. He was the Managing Director of LEI (2019-2024). He is conjoint Professor of Ophthalmology at UWA and the University of Udayana, Indonesia, an adjunct Professor of Ophthalmology at the University of Indonesia and a Consultant Ophthalmologist at Royal Perth Hospital.
Professor Stephen Cringle
Stephen Cringle graduated in physics from Portsmouth Polytechnic (UK) and was later awarded a PhD in ocular physiology from UWA. He is currently a Professor within the UWA Medical School, Centre for Ophthalmology and Visual Science (affiliated with LEI) and is based at LEI.
Other researchers
All staff in LEI’s Physiology and Pharmacology research group made significant contributions to this project, particularly Professor Er-Ning Su, Mr Dean Darcey, Associate Professor Paula Yu, Professor Ian Constable AO, Professor Xinghai Sun, Associate Professor Philip House, Professor Wenyi Guo, and Associate Professor Xiabo Yu.
Partner
This case study was developed with input from Professors Dao-Yi Yu AM, Bill Morgan and Steve Cringle and in partnership with the Lions Eye Institute and the University of Western Australia.

Disclaimer
NHMRC does not endorse or recommend any commercial products, processes, or services. The views and opinions expressed within NHMRC case studies are not necessarily those of the Australian Government. The case studies are not to be used for advertising or product endorsement purposes.
References
The information and images from which impact case studies are produced may be obtained from a number of sources including our case study partner, NHMRC’s internal records and publicly available materials. Key sources of information consulted for this case study include:
1 Davuluru SS, Jess AT, Kim JSB, Yoo K, Nguyen V, Xu BY. Identifying, Understanding, and Addressing Disparities in Glaucoma Care in the United States. Transl Vis Sci Technol. 2023 Oct 3;12(10):18
2 Keel S, Xie J, Foreman J, Lee PY, Alwan M, Fahy ET, van Wijngaarden P, Gaskin JC, Ang GS, Crowston JG, Taylor HR. Prevalence of glaucoma in the Australian national eye health survey. British Journal of Ophthalmology. 2019 Feb 1;103(2):191-5
3 Leffler CT, Pierson K. The origin of the term glaucoma: owls or light-colored eyes? Journal of Glaucoma. 2017 Jun 1;26(6):e197-8
4 Traverso CE, Carassa RG, Fea AM, Figus M, Astarita C, Piergentili B, Vera V, Gandolfi S. Effectiveness and safety of xen gel stent in glaucoma surgery: a systematic review of the literature. Journal of Clinical Medicine. 2023 Aug 16;12(16):5339
5 These figures were provided to LEI by Allergan and were current in January 2023
6 Schlenker MB, Gulamhusein H, Conrad-Hengerer I, Somers A, Lenzhofer M, Stalmans I, Reitsamer H, Hengerer FH, Ahmed II. Efficacy, safety, and risk factors for failure of standalone ab interno gelatin microstent implantation versus standalone trabeculectomy. Ophthalmology. 2017 Nov 1;124(11):1579-88
7 Scheres LM, Kujovic‐Aleksov S, Ramdas WD, de Crom RM, Roelofs LC, Berendschot TT, Webers CA, Beckers HJ. XEN® gel stent compared to PRESERFLO™ MicroShunt implantation for primary open‐angle glaucoma: two‐year results. Acta ophthalmologica. 2021 May;99(3):e433-40
8 Arnould L, Balsat E, Hashimoto Y, White A, Kong G, Dunn H, Fan L, Gabrielle PH, Bron AM, Creuzot-Garcher CP, Lawlor M. Two-year outcomes of Xen 45 gel stent implantation in patients with open-angle glaucoma: real-world data from the Fight Glaucoma Blindness registry. British Journal of Ophthalmology. 2024 May 24
9 Fea AM, Durr GM, Marolo P, Malinverni L, Economou MA, Ahmed I. XEN® gel stent: a comprehensive review on its use as a treatment option for refractory glaucoma. Clinical Ophthalmology. 2020 Jun 30:1805-32
10 Gedde SJ, Feuer WJ, Lim KS, Barton K, Goyal S, Ahmed II, Brandt JD, Banitt M, Budenz D, Lee R, Palmberg P. Postoperative complications in the primary tube versus trabeculectomy study during 5 years of follow-up. Ophthalmology. 2022 Dec 1;129(12):1357-67
11 Traverso CE, Carassa RG, Fea AM, Figus M, Astarita C, Piergentili B, Vera V, Gandolfi S. Effectiveness and safety of xen gel stent in glaucoma surgery: a systematic review of the literature. Journal of Clinical Medicine. 2023 Aug 16;12(16):5339
12 Yu DY, Morgan WH, Sun X, Su EN, Cringle SJ, Paula KY, House P, Guo W, Yu X. The critical role of the conjunctiva in glaucoma filtration surgery. Progress in retinal and eye research. 2009 Sep 1;28(5):303-28