The fundamental framework for the ethical, humane and responsible care and use of animals for scientific purposes in Australia includes the application of the 3Rs – Replacement, Reduction and Refinement of animal use. This Information Paper provides information about the implementation of the 3Rs in Australia. For further information, please refer to the webpage about the 3Rs.
Publication Data
Table of contents
Key terms
| Key term | Definition |
|---|---|
| 3Rs | Replacement, reduction and refinement of animal use |
| Alternative | There is broad international agreement about the definitions of the terms replacement, reduction and refinement. However, the term alternative is sometimes used to refer to replacement of animal use alone or to encompass replacement alternatives, reduction alternatives and refinement alternatives as a whole. For the purposes of this document, the terms replacement, reduction and refinement are as defined in this section and the term alternative is not used to describe all three concepts collectively |
| Animal | As defined in the Code: Any live non-human vertebrate (that is, fish, amphibians, reptiles, birds and mammals, encompassing domestic animals, purpose-bred animals, livestock, wildlife) and cephalopods |
| Animal ethics committee | As defined in the Code: A committee constituted in accordance with the terms of reference and membership laid down in the Code |
| BPM Guidance | Best practice methodology in the use of animals for scientific purposes (2017) (updated 2018) |
| Code | Australian code for the care and use of animals for scientific purposes (2013)1 |
| Institution | As defined in the Code: Any organisation or agency involved in the care and use of animals for scientific purposes, including universities, hospitals, research institutes, government departments, teaching organisations (including schools and colleges), vocational training organisations, agricultural organisations, commercial companies, and organisations involved in animal breeding and supply |
| Investigator | As defined in the Code: Any person who uses animals for scientific purposes. Includes researchers, teachers, undergraduate and postgraduate students involved in research projects, and people involved in product testing, environmental testing, production of biological products and wildlife surveys |
| NHMRC Act | National Health and Medical Research Act 1992 |
| Reduction | Methods for obtaining comparable levels of information from the use of fewer animals in scientific procedures or for obtaining more information from the same number of animals |
| Refinement | Methods that alleviate or minimise potential pain and distress, and enhance animal wellbeing |
| Replacement | Methods that permit a given purpose of an activity or project to be achieved without the use of animals |
| Research | As defined in the Australian code for the responsible conduct of research (2018)2: the concept of research is broad and includes the creation of new knowledge and/or the use of existing knowledge in a new and creative way so as to generate new concepts, methodologies, inventions and understandings. This could include synthesis and analysis of previous research to the extent that it is new and creative |
| Scientific purposes | As defined in the Code: All activities conducted with the aim of acquiring, developing or demonstrating knowledge or techniques in all areas of science, including teaching, field trials, environmental studies, research (including the creation and breeding of a new animal line where the impact on animal wellbeing is unknown or uncertain), diagnosis, product testing and the production of biological products |
| Teaching activities | As defined in the Code: Any action or group of actions undertaken with the aim of achieving a scientific purpose, where the scientific purpose is imparting or demonstrating knowledge or techniques to achieve an educational outcome in science, as specified in the relevant curriculum or competency requirements |
Acronyms
| Acronym | Expanded description |
|---|---|
| AEC | Animal ethics committee |
| ARC | Australian Research Council |
| AWC | Animal Welfare Committee of the National Health and Medical Research Council |
| CSIRO | Commonwealth Scientific and Industrial Research Organisation |
| NHMRC | National Health and Medical Research Council |
| UA | Universities Australia |
Introduction
Background
The Australian community expects any care and use of animals for scientific purpose to be responsible, ethical and humane. The use of animals has led to advances from which all Australians have benefited, such as understanding and treatment of infectious diseases and organ transplantation. Nevertheless, all those involved with the care and use of animals for scientific purposes have a responsibility to ensure the consideration and implementation of alternative approaches that do not use animals. If the use of animals is the only way to obtain the necessary information, there is a responsibility to ensure that the studies are of the highest quality, designed to involve the fewest necessary number of animals and to safeguard animal wellbeing. These principles known as the 3Rs — Replacement, Reduction and Refinement — were first proposed by William Russell and Rex Burch in 1959.3
The 3Rs are now recognised internationally as the framework for the ethical and humane use of animals for scientific purposes and have become embedded in national and international legislation and regulation, and policies of organisations that fund or conduct animal research.
| Replacement | Methods that permit a given purpose of an activity or project to be achieved without the use of animals. |
|---|---|
| Reduction | Methods for obtaining comparable levels of information from the use of fewer animals in scientific procedures or for obtaining more information from the same number of animals. |
| Refinement | Methods that alleviate or minimise potential pain and distress and enhance animal wellbeing. |
In Australia, the state and territory governments are responsible for the regulation of animal welfare, including the care and use of animals for scientific purposes. The Australian code for the care and use of animals for scientific purposes (2013) (the Code)1 is adopted in legislation in all Australian states and territories.
The Code is published by the National Health and Medical Research Council (NHMRC) and endorsed by NHMRC, the Australian Research Council (ARC), the Commonwealth Scientific and Industrial Research Organisation (CSIRO) and Universities Australia (UA). It sets out the framework for the ethical, humane and responsible care and use of animals for scientific purposes and provides guidance for institutions, animal ethics committees (AECs) and investigators. The Code applies to the care and use of all live non-human vertebrates and cephalopods.
The governing principles in the Code include the necessity for the application of the 3Rs at all stages of animal care and use. These principles underpin the requirements in the entire Code including the responsibilities of institutions, animal ethics committees, investigators and animal carers.
The 3Rs are established in Australia through adoption of the Code in state and territory legislation.
In addition to underpinning the framework for the ethical and humane use of animals, the 3Rs are recognised as providing a structure and a tool for the conduct of high quality animal-based studies and the application of good scientific method. The Best practice methodology in the use of animals for scientific purposes, 2017 (updated July 2018) (BPM Guidance)4 provides guidance for the conduct of high-quality animal-based studies, including the application of the 3Rs. This guidance was developed by NHMRC and is supported by ARC, CSIRO and UA. It is intended to support the implementation of the Code.
NHMRC and the 3Rs
NHMRC is the leading agency for funding health and medical research in Australia. NHMRC’s purposes are to fund high-quality health and medical research and build research capability, support the translation of health and medical research into better health outcomes and promote the highest standards of ethics and integrity in health and medical research. These purposes support NHMRC’s mission of ‘building a healthy Australia’. They reflect the role for NHMRC set out in its enabling legislation – the National Health and Medical Research Council Act 1992 (NHMRC Act).
NHMRC does not fund research involving animals unless the work is:
- of high quality and significance as determined by NHMRC's peer review process
- ethically reviewed and approved by an AEC before the work begins, and
- conducted in accordance with relevant legislation and the Code, which includes strict requirements for the application of the 3Rs.
For institutions and researchers receiving NHMRC funds, NHMRC's expectations are that:
- Institutions promote compliance with the Code with respect to the 3Rs, including the development and promotion of relevant policies and procedures and the provision of training and resources (Clause 2.1.5 [iii] of the Code).
- Researchers apply the principles of the 3Rs at all stages of animal care and use including planning, conducting and reviewing projects, as outlined in the Code.
NHMRC’s 3Rs Project
Despite the importance of the 3Rs, there is limited documented evidence about the use of the 3Rs in Australia. To address this information gap, NHMRC’s Animal Welfare Committee has overseen a project to obtain current information about how the 3Rs are being implemented in Australia and factors that enable or hinder their development and adoption. The project’s scope did not include funding of projects for the development of the 3Rs, identification of prospective research areas for the 3Rs, and the benefits or otherwise of specific 3R methods or techniques.
Major components of the project included the conduct of a literature review and a survey of those involved with the use of animals for scientific purposes in Australia (the 3Rs Survey).
Aim of this document
This document is intended to present information about the implementation of the 3Rs in Australia, to promote informed discussion of the issues and guide recommendations for improvement if required.
To this end, NHMRC provides this Information Paper for broad circulation within the Australian community. It will be of particular interest to those directly involved with the care and use of animals for scientific purposes – institutions, investigators, AEC members and animal carers — as well as animal advocacy groups, professional groups, veterinarians, and those involved with policy and legislation related to the care and use of animals for scientific purposes.
This Information Paper is not intended to represent actions that will be taken by NHMRC, as implementation of the 3Rs in Australia is the responsibility of the sector as a whole. Many areas for potential improvements that have been identified are not within NHMRC’s remit under the NHMRC Act.
Structure
This paper is structured to provide information in seven areas.
- Section 1 outlines the importance of the 3Rs to the conduct of high-quality animal-based studies.
- Section 2 summarises background information about the literature review and the 3Rs Survey.
- Section 3 highlights how innovations in the 3Rs can be unrecognised.
- Section 4 presents an overview of the implementation of the 3Rs in Australia and identifies strengths and opportunities for consideration by the sector.
- Section 5 provides advice about some international 3Rs centres that can be a source of information about current, and the development of new, 3Rs methods and techniques.
- Section 6 outlines how this paper was developed.
- Section 7 provides information about relevant publications and further resources.
1. The 3Rs and ensuring high-quality animal-based studies
This section discusses the principles and implementation of the 3Rs and outlines the significance of the 3Rs to the conduct of high-quality animal-based studies.
Introduction
The 3Rs underpin the framework for the ethical and humane use of animals and must be considered at all stages of animal care and use, including the planning, conduct and review of projects (Code, Clause 1.1 [v]). In addition, the 3Rs are recognised as providing a structure and a tool for the conduct of high quality animal-based studies and the application of good scientific method. 5-8 Further guidance about the significance of the 3Rs is outlined in the BPM Guidance.4
Replacement
Application of the principle of replacement involves the use of methods that allow the aims of a project to be achieved without the use of animals in all or part of the study (Code, Clauses 1.18–1.20). Techniques to replace the use of animals include the use of epidemiological data; physical and chemical analysis; computer, mathematical and inanimate synthetic models; simulations; in vitro systems; non-sentient organisms; cadavers; and clinical cases (Code, Clause 1.19).
The planning phase of a study should involve identification of all feasible methods of testing the study’s hypotheses including viable non-animal models and the use of less sentient alternatives such as invertebrates. Systematic review of animal-based studies should be considered where appropriate. 9-16 The validity and relevance of a proposed animal model must be assessed. If there is insufficient evidence to support the validity of an animal model, its use must be rejected.
Reduction
Application of the principle of reduction enables the proposed aim(s) of the study to be achieved from fewer animals, whilst ensuring that sufficient numbers of animals are used to satisfy good statistical design (Code, Clauses 1.21–1.27).17 The use of too few animals may lead to invalid or low quality results and wastage of animals, and the unnecessary use of animals in future studies that build on invalid results. The use of too many animals is a potential unnecessary ethical cost to animals and waste of resources. Repetition of experiments to provide assurance about the validity of the observed effect must be essential for the study’s statistical design (Code, Clause 1.23).
Application of the principle of reduction also enables more information to be obtained from a given number of animals so that fewer animals are used overall. However, reduction should not result in greater harm, including pain and distress, to the animals used (Code, Clause 1.24).
Refinement
Application of the principle of refinement involves the use of methods that avoid or minimise potential harm, including pain and distress, to the animals and enhance animal wellbeing (Code, Clauses 1.8–1.14 and 1.28–1.30). Animals with compromised wellbeing have disturbed behaviour, physiology and immunology that can lead to unreliable conclusions and/or unwanted variation in scientific output, affecting the reliability and reproducibility of studies.18 Refinement applies to all aspects of the care and use of animals, including their care and management as well as methods employed during their use.
2. Literature review and 3Rs Survey
This section provides background information about the literature review and the 3Rs Survey conducted as part of NHMRC’s 3Rs project.
Literature review
The aim of the literature review was to obtain background information about the uptake, acceptance and implementation of the 3Rs in Australia and overseas, the enablers and barriers to implementing the 3Rs and knowledge gaps about the 3Rs.
The scope of the literature review was linked to the scope of the Codea – in particular, the definitions of animal and scientific purposes in the Code (see Key terms) – and was not limited to health and medical research or NHMRC-funded research. Specific 3Rs techniques and methods used in individual studies were beyond the scope of this review.
The literature review examined peer-reviewed publications from the period 2007–2017, supplemented with a review of the ‘grey’ literature comprising the website content of Australian government agencies and universities, the website content of several Australian and international organisations and an audit of animal research ethics applications used in Australian universities.b
The University of Tasmania was commissioned to conduct the literature review on behalf of NHMRC.
The outcomes of the literature review were used to inform the design of the 3Rs Survey.
The 3Rs Survey
International surveys about the 3Rs have sought the views of researchers, AEC members, animal welfare officers, and licensing authorities.19-27 Both qualitative in-depth interviews and quantitative survey methods have been used. There have been few international surveys of institutional representatives. Australian surveys have targeted specific groups such as animal welfare officers or focused on specific aspects of the care and use of animals for scientific purposes (e.g. education and training29).
To gain a broader insight into how the 3Rs are currently understood, considered and implemented in Australia, the views and advice were sought from those directly involved:
- Investigators who were involved with the use of animals for scientific purposes in Australia sometime during the last three years.
- Current members of Australian AECs.
- Australian institutional representatives – senior people within institutions who were responsible for overall institutional governance with respect to the care and use of animals for scientific purposes.
The scope of the survey reflected the scope of the Codea and was not restricted to health and medical research or NHMRC-funded research.
To ensure independence of the survey process and the anonymity of participants, ORIMA Research, an independent market and social research company, was commissioned to conduct the survey on behalf of NHMRC. The Australian Government Department of Health Human Research Ethics Committee approved the survey, which was conducted between 5 March 2018 and 28 May 2018 using an online questionnaire.
The survey content and design were informed by peer-reviewed literature, international examples and relevant requirements in the Code. Further, the survey was designed to enable comparison of responses between the different target groups where possible, and comparison of key survey results with data obtained about other countries — in particular, data from surveys conducted in the United Kingdom in 200819, Denmark in 201620 and Europe in 201121 where the major participants were researchers.
Because of privacy constraints, NHMRC managed the distribution of survey invitations to potential participants. Senior staff in NHMRC-funded institutions and investigators named on NHMRC-funded projects received invitations directly from NHMRC. Survey invitations were also provided to third parties (state and territory government departments responsible for animal welfare in each Australian jurisdiction and staff of Research Administration Offices in NHMRC-funded institutions) for onward distribution to further members of the target groups.
The 3Rs Survey Report is available from NHMRC’s website.30
Comment about interpretation
Interpretation of the results from the 3Rs Survey, and comparison with data obtained about other countries, must take into account:
- the inevitable changes in views and practices since the time each survey was conducted (Australia 2018, Denmark 2015, UK 2008), and
- the differences in the target groups in each survey and the respondents’ fields of animal use.
3. Unrecognised innovations in the 3Rs
Background
In Australia, there may be more innovations in the replacement, reduction and refinement of animal use than are actually visible. This position has also been identified in the UK and the Netherlands.31,32 Unrecognised 3R development and implementation can occur in situations where the primary purpose of the study was not the development of a 3R method or technique. For example:
- Within projects where changes are made to in vitro models, in animal models or in production processes that, directly or indirectly, lead to a reduction or refinement of laboratory animal use.
- Projects where the primary aim is not the development of a 3Rs method or technique, but may indirectly lead to the development of methods that replace, reduce or refine the use of animals.
- Projects where the use of animals was required to achieve the stated aim, but are now conducted without using animals in all or part of the study.
If this ‘incidental’ 3Rs development and implementation is not recognised and/or is not cited as such in the publications arising from the studies, this information is difficult to find.
Innovations in the 3Rs can also be unrecognised in situations where information is not published; for example:
- 3R research where the outcome is valid results that are either negative or neutral in nature
- competitive research involving commercial confidentiality and intellectual property.
It should be noted that, in Australia, AEC approval is required before the use of animals for scientific purposes may commence. In addition, information about the use of animals is provided in reports to AECs and to state and territory governments. These reports may contain information about the implementation of the 3Rs in AEC approved projects. For new projects where animal use has been replaced with non-animal alternatives, investigators are not required to obtain AEC approval for the work or report about animals that they have never planned to use. The AEC approval and animal use reporting systems are therefore not useful for the identification of situations where the use of animals has been replaced.
Examples
There are many examples of projects conducted in Australia, including NHMRC-funded research, where the outcomes contribute to the 3Rs, but may not be recognised as such. These include:
- Collaborative work between laboratory-based researchers and mathematical modellers where computer simulation is leading to improvements in the design of studies using animals, so that the minimum numbers of animals are used to produce useful high-quality data.33,34 NHMRC funding has contributed to this work.
- The development of organoids — miniature organs derived from human cells and grown in culture dishes — that allow studies outside a human or animal and replace the use of animals.35 Their use includes the investigation of biological processes such as cell behaviour, tissue repair and response to drugs or mutations; organ function; modelling of diseases such as epilepsy, autism, Alzheimer’s disease and Parkinson’s disease and a range of cancers; gene editing; and transplantation.
Specific examples of organoids developed in Australia to replace the use of animals:
- Kidney organoids that represent powerful models of the human organ for applications including nephrotoxicity screening, disease modelling and as a source of cells for therapy.36-38 This work was funded by NHMRC.
- Models of human heart tissue that enable the study of cardiac biology and diseases ‘in a dish’. The viable, functioning human heart muscle allows researchers to model disease, screen new drugs and investigate heart repair following injury without the use of animals.39,40 NHMRC funding has contributed to this work.
- The development of a ‘brain in a box’ — a 3D network of connected neurons made from a patient’s cells — that will be used to test medications for brain disorders so the most suitable drug and dose can be found. In addition to the 3D scaffold of neurons, the design includes electrodes that will stimulate artificial seizures in the ‘brains’ and record electrical activity to test if drugs are effective.41 NHMRC funding has contributed to this work.
- The use of cancer tumours sourced from humans and grown in a gelatin sponge platform in the laboratory instead of in mice to investigate new cancer therapies.42 NHMRC funding has contributed to this work.
- The use of a newly developed assay to assess a large cohort of over 20 different HIV-1 poxvirus vaccination strategies in mice, with a marked reduction (>100-fold) in the number of animals used.43 This work was funded by NHMRC.
- Establishment of a more efficient and effective method to characterise anti-tumour immunity when assessing cancer immunotherapies in mouse models, which also resulted in a significant reduction of mice used.44
- Use of dogs with naturally occurring cancer similar to humans for trials of cancer treatments prior to human clinical trials. This replaces the induction of the disease in a rodent model for pre-clinical testing of cancer treatments prior to human clinical trials.45 This work was funded by NHMRC.
4. The 3Rs in Australia – Current practices, strengths and opportunities
This section presents an overview of the implementation of the 3Rs in Australia and views about key enablers and barriers to their implementation, drawing primarily on the findings from the 3Rs Survey.30 Areas of strength as well as potential opportunities for improvement are identified for consideration by the sector.
Information in this section does not represent recommended actions that will be taken by NHMRC as implementation of the 3Rs in Australia is the responsibility of the sector as a whole. Many areas for potential improvements identified in this paper are not within NHMRC’s remit under the NHMRC Act.
Current practices
Literature review
The literature review highlighted the paucity of peer-reviewed publications during the period 2007–2017 about the acceptance, uptake and/or implementation of the 3Rs in Australia.
Internationally, the majority of articles in the peer-reviewed literature published in the period 2007–2017 addressed the topic of replacement. The review highlighted the breadth of information available about 3Rs implementation in toxicity testing and product development. The results also indicated that the uptake of the 3Rs varies across countries and according to the regulatory and legislative environment in which the research is conducted.
The outcome from the review of the ‘grey’ literature was current at the time of its completion in August 2017, and the content of relevant websites and animal ethics application forms may have since been updated. However, the following key findings may still be indicative of the current situation and trends.
- The 3Rs are referenced on 37% of Australian university websites.
- There are no Australian universities with a publicly available 3Rs strategy.
- The 3Rs are referenced in 54% of university research/ animal research policies.
- Most jurisdictions (75%) referred to the 3Rs in guidance provided for AECs.
- 78% of AEC application forms used in Australian universities include explicit reference to all the 3Rs.
Although, these findings suggest that the 3Rs may not have been fully implemented and adopted in Australia, they do not provide a detailed insight into the extent to which Australian investigators, AEC members and institutional representatives understand, consider and/or support and implement the 3Rs.
3Rs Survey
The 3Rs Survey Report30 provides details about the 3Rs in Australia in the following areas:
- knowledge and understanding of the 3Rs
- attitudes about the 3Rs
- enablers and barriers to the implementation of the 3Rs
- 3Rs in practice including when and how the 3Rs are considered and the role they play, deciding about the number of animals used, pilot studies and institutional support for the 3Rs
- information access including sources of information and problems encountered
- education and training about the 3Rs including the nature, frequency and effectiveness of training and participation in training, and
- communication about the 3Rs including dissemination of information by investigators and promotion of the 3Rs by institutions.
The 3Rs Survey response rate and the respondent profiles suggest that the results provide insights about views and practices across the breadth of animal use in Australia.
Knowledge and understanding
Lack of awareness and insufficient 3Rs knowledge and expertise were identified in peer-reviewed literature as a barrier to 3Rs implementation.
The 3Rs Survey demonstrated that investigators and AEC members in Australia appear to have a long-standing awareness of the 3Rs.
The 3Rs Survey assessed factual understanding of the 3Rs amongst investigators and AEC members by presenting respondents with a set of definitions for replacement, reduction, and refinement. These definitions included correct statements as well as some common misconceptions. To allow for comparison with international datasets, the descriptions were consistent with those used in comparable surveys in the UK and Denmark. Respondents were asked to identify all of the definitions that aligned with their understanding of each of the 3Rs.
Factual understanding of the 3Rs appears to be strongest in the area of replacement, with misconceptions most common in the area of refinement. The results for investigators were generally consistent with those from comparable surveys in the UK and in Denmark (see Figure 1). AEC members tended to demonstrate a stronger understanding of the 3Rs compared to investigators, particularly about refinement. Nevertheless, a substantial number of investigators (similar to comparable countries) and AEC members selected incorrect definitions of reduction and refinement.
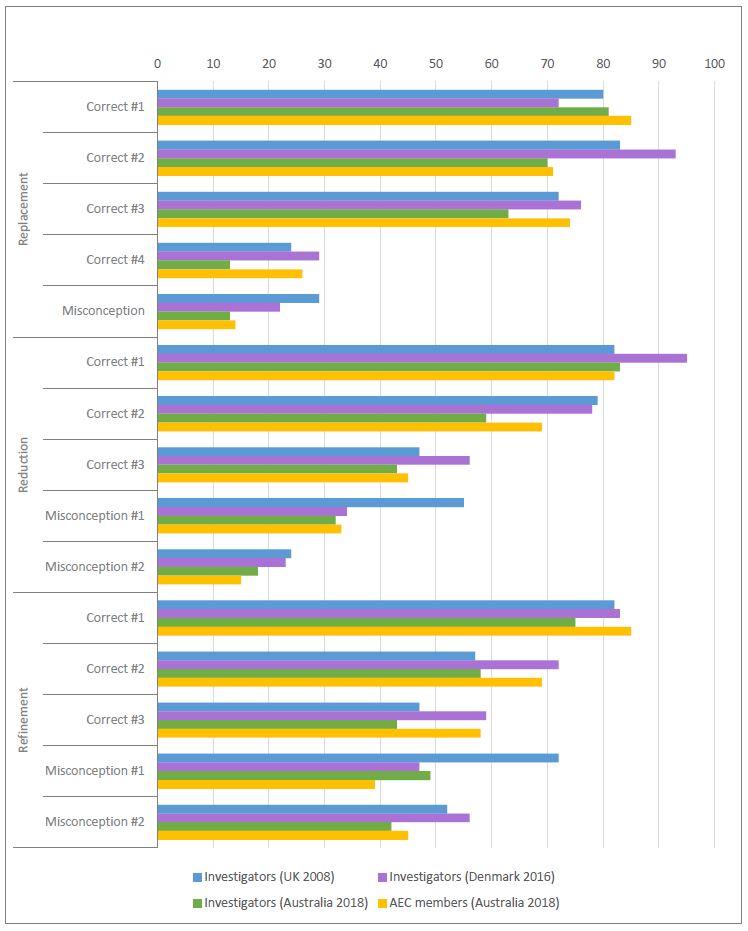
Figure 2 shows the proportion of investigators and AEC members in Australia who selected only one or more of the correct definitions of each of the 3Rs and did not select any of the statements that represented a misconception.
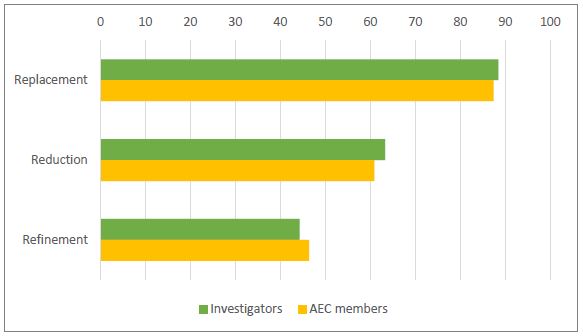
Areas of poor understanding
Key areas of poor understanding amongst investigators and AEC members in Australia, reflected by the low proportion selecting the relevant correct definition, are outlined in Table 1.
| Area | Definition | % selecting this definition in Australia |
|---|---|---|
| Reduction | Reducing the number of animals used per experiment | 59% of investigators (which is relatively low when compared to 79% of investigators in the UK and 78% of investigators in Denmark). |
| Reduction | Obtaining more information from an experiment while using the same number of animals | 43% of investigators and 45% of AEC members. |
| Refinement | Improving the conditions in which animals are kept | 43% of investigators |
Of the two correct definitions of Replacement, Replacing vertebrates with invertebrates was selected by only 13% of investigators and 26% of AEC members in Australia which may reflect a low awareness and/or the use of invertebrate models such as insects in Australia.
The majority of investigators participating in the 3Rs Survey reported using mice (69%) and rats (28%) with 17% reporting the use of livestock, 3%–7% reporting the use of other vertebrate species identified in the survey, none reporting the use of cephalopods, and 4% reporting the use of ‘other species’.
Comparison of this result with those from comparable international surveys should also be interpreted in light of the definition of ‘animal’ in relevant legislation at the time of the surveys. For example, cephalopods are covered under the definition of animal in the Code. At the time of the UK Survey in 2008, the UK Animals (Scientific Procedures) Act 1986 did not cover the use cephalopods.c At the time of the Danish survey in 2016, the Danish Order of the Animal Testing Act 2013 covered the use of cephalopods.d
Attitudes
The findings from the literature review indicated that acceptance of the 3Rs in research is generally improving. Acceptance is influenced by social and cultural factors, the type of animal involved in the study and individual attitudes.
The 3Rs Survey indicated that most investigators and AEC members agreed that 3Rs methods are recognised throughout the Australian scientific community.
The survey results demonstrated some differences in attitudes between investigators involved in different fields and at different levels of experience, and between investigators and AEC members. Interestingly, the attitudes amongst investigators in Australia about aspects of the use of animals were generally comparable to those amongst investigators in the UK and/or Denmark (see Figures 3 and 4).
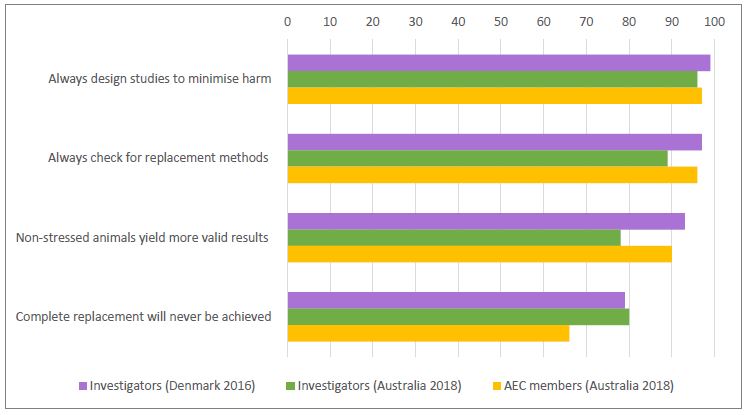
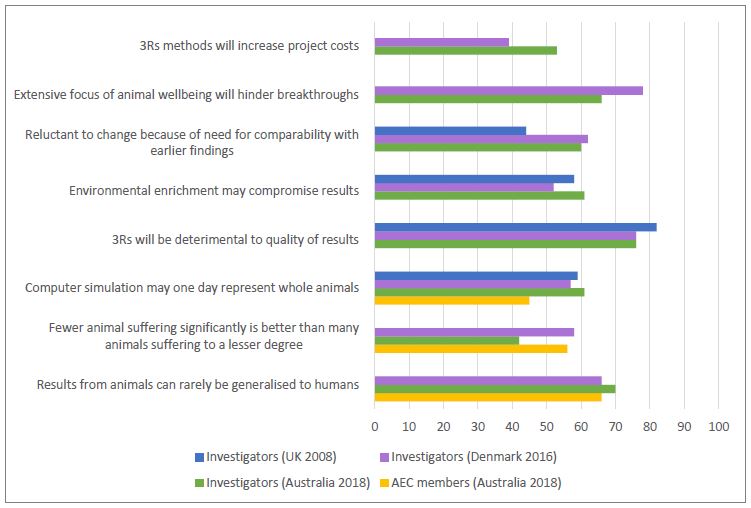
Enablers and barriers
Literature review
The literature review identified 11 enablers and supports for the uptake and implementation of the 3Rs. In the main, barriers to the implementation of the 3Rs identified in the literature review were the converse of the enablers:
- Legislation and regulation
- Guidelines and standards
- Education and training
- Leadership and governance
- Improved reporting of experiments in publications
- Databases and information sharing
- Ethics committee processes
- Industry involvement and collaboration
- Research support
- Policies and frameworks
- Workforce roles.
There were few peer-reviewed articles about enablers and barriers to the implementation of the 3Rs in Australia.
3Rs Survey
The results from the 3Rs Survey highlighted the views of investigators, AEC members and institutional representatives about factors that promote or hinder the implementation of the 3Rs in Australia. Full details are provided in the 3Rs Survey Report.30 The report also highlights differences in the factors reported by the different participants groups and the influences of the profile of the participants – in particular, the type of activity they were involved in where animals were used.
Enablers and supports
Key factors identified as enablers of the implementation of the 3Rs are summarised in Table 2.
| Replacement | Reduction | Refinement |
|---|---|---|
|
|
|
Additional key points highlighted in the survey results include:
- Investigators and AEC members identified the AEC review process as the top driving factor for using 3R methods. Personal ethos was another key driving factor for investigators, while AEC members felt that legislation was a key driving factor for the use of the 3Rs.
- The strategy that investigators and AEC members felt would most effectively support and facilitate the implementation of the 3Rs was education and training focused on the 3Rs. Strategies and initiatives perceived to be the least effective were financial support and public recognition of the implementation and use of the 3Rs.
- Just over a third of investigators felt that nothing would enable them to achieve their objectives without using animals, as their work demands that they look at the whole animal system.
- While about half of AEC members identified greater willingness amongst investigators to change their methods as an enabler for refinement, most investigators (60%) disagreed with the statement that they were ‘reluctant to change the way they work because of need for comparability with earlier findings’.
Barriers
All participant groups identified the lack of appropriate scientific or technological innovation as the primary barrier to implementation of the 3Rs. Other key barriers included comparability of data (identified by investigators) and insufficient funding available (identified by institutional representatives). A significant difference was observed in the perceived level of importance of the pressure of time or other duties for investigators as a barrier to implementation of the 3Rs, with this factor reported as a barrier by only a small number of investigators compared to a relatively higher number of AEC members and institutional representatives.
Of note, approximately one-third of investigators and institutional representatives reported that there were no barriers to implementing the 3Rs.
Areas of strength
The implementation of the 3Rs appears to be strongest in the following areas:
- Awareness and knowledge of the 3Rs amongst investigators and AEC members – for investigators, at levels comparable to other countries.
- Consideration of the 3Rs by investigators during the design, conduct and review of animal care and use. Given the role of the 3Rs in ensuring high-quality animal-based studies, the finding that 90% of investigators reported that they consider the 3Rs when designing an experiment is extremely positive.
- Use of power calculations by investigators when generally deciding on the number of animals to use.
- Reported development of original 3Rs techniques by investigators during the five years prior to the conduct of the survey.
- The animal ethics committee review process as a key driving factor for using 3R methods, along with the personal ethos of investigators.
- Consideration of the 3Rs by AEC members when reviewing an application.
- Access to education and training about the 3Rs provided by institutions, including induction and refresher training.
- Communication about the 3Rs by investigators during work meetings.
Potential opportunities
The following information about potential opportunities for improvement are provided for consideration by the sector. This information does not represent recommended actions that will be taken by NHMRC as implementation of the 3Rs in Australia is the responsibility of the sector as a whole. Many areas for potential improvements identified in this paper are not within NHMRC’s remit under the NHMRC Act.
- Provision of publicly available information by institutions about their policies and strategies about the 3Rs.
- Promotion and championing of the 3Rs by institutions.
- Effective education, training and information access to increase awareness, knowledge and expertise about the 3Rs amongst investigators and AEC members.
The Code includes mandatory requirements related to competency (Clause 1.29). The term ‘competent’ is also defined in the Code.e Given that the governing principles in the Code require the application of the 3Rs at all stages of animal care and use, and the role played by the 3Rs in ensuring high quality animal-based studies, ensuring that people are competent in the application of the 3Rs, supported by institutional education and training about the 3Rs, may be reasonable. - Access to information about the availability of current, and the development of new, 3Rs methods and techniques. Examples include improved availability of information resources and search tools, and promotion of resources and information provided by international 3Rs centres (see Section 5). The need for a 3Rs centre in Australia was not explored in the survey. However, science is an international activity and the large body of international literature about the 3Rs may be readily applicable to the Australian environment. Tools for the dissemination of ideas and new knowledge, and access to information, are rapidly advancing. Those involved with the care and use of animals for scientific purposes should be supported and encouraged to find and make use of new 3Rs knowledge and approaches developed by the international community, including that promulgated by 3Rs centres in other countries.
- Institutional assistance to investigators to identify relevant 3R methods and techniques at all stages of animal care and use, in particular, during the design of studies.
- Where appropriate, use of pilot studies to test a hypothesis, a model or a method before the larger scale study is planned and performed. It should be noted that the use of pilot studies is not relevant to every field where animals are used.
- Availability of expert advice from a statistician for investigators and AEC members about the statistical design of a study or the optimal number of animals to be used.
- Discussion about the use of non-animal alternatives and choice of animal species during consideration of an application by AEC members.
- Reporting and dissemination of the development and use of 3Rs techniques and methods, including publication of null or neutral results, compliance with reporting guidelines such as the ARRIVE Guidelines46, and the recognition of incidental development of 3R methods and techniques with use of appropriate methods to identify these innovations in publications.
Potential opportunities for further action include:
- Use of the 3Rs Survey results as a baseline for measurement of change over time. Repeat and/or follow-up surveys may allow an evaluation of the effectiveness of any strategies implemented.
- Qualitative surveys or interviews may allow a more in-depth exploration of the views and practices about the 3Rs to inform ongoing improvements.
- Survey focused specifically on those involved with the use of animals in teaching activities. The international surveys used to inform the content of the 3Rs
Survey focussed on the use of animals in activities other than teaching. Some of the survey questions were therefore not suited for animal use in teaching activities, such as in primary and secondary schools and undergraduate teaching. However, those involved with the use of animals in teaching activities were encouraged to provide their views for questions that applied to their situation. Nevertheless, further exploration of the views and practices of this sector may be useful.
5. International 3Rs Centres
The following are examples of some international 3Rs Centres that can be a source of information about current, and the development of new, 3Rs methods and techniques.
| Centre | Website |
|---|---|
| Canadian Council on Animal Care. Three Rs Microsite - Three Rs Searches & Animal Index | https://3rs.ccac.ca/en/searches-and-animal-index/ |
| Danish 3R-Centre | https://3rcenter.dk/ |
| EU Reference Laboratory for alternatives to animal testing (EURL ECVAM) | https://ec.europa.eu/jrc/en/eurl/ecvam |
| German Centre for the Documentation and Evaluation of Alternative Methods to Animal Experiments (ZEBET) | https://www.bfr.bund.de/en/about-us/bfr-structure/department-experimental-toxicology-and-zebet/evaluation-of-alternative-methods-to-animal-experiments/ |
| Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) | https://ntp.niehs.nih.gov/pubhealth/evalatm/iccvam/index.html |
| Johns Hopkins University Center for Alternatives to Animal Testing (CAAT) | http://caat.jhsph.edu/ |
| National Centre for the Replacement Refinement and Reduction of Animals in Research (NC3Rs) (UK) | https://www.nc3rs.org.uk/ |
| Norwegian Consensus-Platform for Alternatives (NORECOPA) | https://norecopa.no/ |
6. Process report and acknowledgements
The 3Rs Project and the development of the Information Paper: Implementation of the 3Rs in Australia (Information Paper) was overseen by NHMRC’s Animal Welfare Committee (AWC). The AWC is established as a working committee under Section 39 of the National Health and Medical Research Council Act 1992 (NHMRC Act) to advise NHMRC on issues pertaining to the conduct and ethics of using animals in biomedical research.
The draft Information Paper developed by the AWC was considered and supported by NHMRC’s Research Committee on 27 June 2019 and Council of NHMRC on 12-13 June and 1 August 2019. Council advised NHMRC’s CEO to release the Information Paper.
Animal Welfare Committee
| Member | Expertise |
|---|---|
| Professor Edna Hardeman | Chair Person with expertise in the use of animals for health and medical research |
| Dr Simon Bain | Person with expertise in veterinary science and the care and use of animals for scientific purposes |
| A/Professor Thomas Burne | Person with expertise in the use of animals for health and medical research |
| Ms Anna Hall | Person with experience in furthering the welfare of animals |
| Dr Mark Lawrie | Person with expertise in veterinary science and the care and use of animals for scientific purposes |
| Ms Robin Matthews | Person with an understanding of community attitudes to the care and use of animals for scientific purposes |
| Dr Carole Webb | Person with experience in furthering the welfare of animals |
Disclosure of interest and management of conflicts of interests
Throughout the 3Rs Project and the development of the Information Paper, disclosure of interests and management of conflicts of interest was undertaken in accordance with the requirements of the NHMRC Act and NHMRC's Policy on the disclosure of interest requirements for prospective and appointed NHMRC committee members. A record of interest was maintained by NHMRC, and relevant information was made publicly available on the NHMRC website to ensure transparency.
Other contributors
NHMRC wishes to acknowledge and thank the contributors of the following organisations and individuals:
- The University of Tasmania: For the conduct of the literature review.
- ORIMA Research: For the conduct of the 3Rs Survey.
For assistance with distribution of invitations to potential participants in the 3Rs Survey:
- state and territory government departments responsible for animal welfare
- staff of Research Administration Offices in NHMRC-funded institutions.
For promotion of the 3Rs Survey:
- Australian and New Zealand Council for the Care of Animals in Research and Teaching
- Australian and New Zealand Laboratory Animal Association
- Australian Research Council
- Australian Veterinary Association
- Commonwealth Scientific and Industrial Research Organisation
- Universities Australia.
NHMRC Project team
- Mary Bate
- Jasmine Witteveen (February 2017 - January 2018)
7. References
1 National Health and Medical Research Council. (2013) Australian code for the care and use of animals for scientific purposes, 8th edition. Canberra: National Health and Medical Research Council.
2 National Health and Medical Research Council. (2018) Australian Code for the Responsible Conduct of Research. Canberra: National Health and Medical Research Council.
3 Russell WMS, Burch, R. (1959) The principles of humane experimental technique. Methuen, London. Accessed from: http://altweb.jhsph.edu/pubs/books/humane_exp/het-toc
4 National Health and Medical Research Council. (2018) Best practice methodology in the use of animals for scientific purposes, 2017 (updated July 2018). Canberra: National Health and Medical Research Council.
5 Graham ML, Prescott MJ. (2015) The multifactorial role of the 3Rs in shifting the harm- benefit analysis in animal models of disease. European Journal of Pharmacology. 759: 19–29. doi:10.1016/j.ejphar.2015.03.040
6 Hawkins D, Gallacher E, Gammell, M. (2013) Statistical power, effect size and animal welfare: recommendations for good practice. Animal Welfare. 22: 339–344. doi:10.7120/09627286.22.3.339
7 Parker RMA, Browne WJ. (2014) The place of experimental design and statistics in the 3Rs. ILAR J. 55(3): 477–485. doi:10.1093/ilar/ilu044
8 Aske K C, Waugh CA. (2017) Expanding the 3R principles. More rigour and transparency in research using animals. EMBO reports. e201744428. doi:10.15252/embr.201744428
9 Hooijmans CR, Leenaars M, Ritskes-Hoitinga M. (2010) A gold standard publication checklist to improve the quality of animal studies, to fully integrate the Three Rs, and to make systematic reviews more feasible. Altern Lab Anim. 38(2): 167–182. Doi:10.1177/026119291003800208
10 de Vries RBM, Hooijmans CR, Langendam MW, van Luijk J, Leenaars M, Ritskes-Hoitinga M, Wever KE. (2015) A protocol format for the preparation, registration and publication of systematic reviews of animal intervention studies. Evidence-based Preclinical Medicine. 2: 1–9, e00007. doi:10.1002/ebm2.7
11 Sena ES, Currie GL, McCann SK, Macleod MR, Howells DW. (2014) Systematic reviews and meta-analysis of preclinical studies: Why perform them and how to appraise them critically. Journal of Cerebral Blood Flow & Metabolism. 34(5): 737–742. doi:10.1038/jcbfm.2014.28
12 Vesterinen HM, Sena ES, Egan KJ, Hirst TC, Churolov L, Currie GL, Antonic A, Howells DW, Macleod MR. (2013) Meta-analysis of data from animal studies: a practical guide. J Neurosci Methods. 221: 92–102. doi:10.1016/j.jneumeth.2013.09.010. Epub 2013 Oct 4.
13 CAMARADES-NC3Rs Preclinical Systematic Review and Meta-analysis Facility (SyRF). Accessed from: http://syrf.org.uk
14 Leenaars M, Hooijmans CR, van Veggel N, ter Riet G, Leeflang M, Hooft L et al. (2012) A step-by-step guide to systematically identify all relevant animal studies. Laboratory Animals. 46(1): 24–31. doi:10.1258/la.2011.011087
15 Collaborative Approach to Meta Analysis and Review of Animal Data from Experimental Studies (CAMARADES). Accessed from: http://www.dcn.ed.ac.uk/camarades/
16 Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE). Accessed from: https://norecopa.no/3r-guide/systematic-review-centre-for-laboratory-animal-experimentation-syrcle
17 Gaines Das R, Fry D, Preziosi R, Hudson M. (2009) Panning for reduction. (2009) ATLA. 37: 27–32. Accessed from: http://www.atla.org.uk/planning-for-reduction/
18 Prescott M, Lidster K. Improving quality of science through better animal welfare: the NC3Rs strategy. (2017) Lab Animal. 46(4): 152–156. doi:10.1038/laban.1217
19 National Centre for the Replacement, Refinement and Reduction of Animals in Research. (2008) Views on the 3Rs. Survey report. Accessed from: https://faunalytics.org/views-on-the-3rs-survey-report-2008/
20 Nøhr R, Lund TB, Lassen J. (2016) The Danish 3R survey: knowledge, attitudes and experiences with the 3Rs among researchers involved in animal experiments in Denmark. Frederiksberg: Department of Food and Resource Economics, University of Copenhagen. (IFRO Report; No. 249). Accessed from: http://en.3rcenter.dk/footer/the-3rs/the-danish-3r-survey/
21 Basel Declaration Society. (2011) Survey: 3R principles in biological and biomedical research laboratories. Accessed from: http://www.basel-declaration.org/projects/3r-report1/
22 Fenwick N, Danielson P, Griffin G. (2011) Survey of Canadian animal-based researchers’ views on the three Rs: Replacement, reduction and refinement. PLoS ONE. 6(8):e22478. doi:10.1371/journal.pone.0022478
23 Kolar R, Ruhdel I. (2007) A survey concerning the work of ethics committees and licensing authorities for animal experiments in Germany. ALTEX. 24(4): 326–34. PMID: 18288429
24 Leenaars M, Savenije B, Nagtegaal A, van der Vaart L, Ritskes-Hoitinga M. (2009) Assessing the search for and implementation of the Three Rs: a survey among scientists. Alternatives to Laboratory Animals. 37(3): 297–303. PMID: 19678731
25 Schuppli C, Fraser D. (2005) The interpretation and application of the three Rs by animal ethics committee members ALTA. 33: 487–500. PMID: 16268760
26 Silverman J, Lidz CW, Clayfield JC, Murray A, Simon LJ, Rondeau RG. (2015) Decision making and the IACUC: Part 1 – Protocol information discussed at full-committee reviews. JAALAS. 54(4): 389–398. PMCID: PMC4521573
27 Van Luijk J, Cuijpers Y, van der Vaart L, de Roo TC, Leenaars M, Ritskes-Hoitinga M. (2013) Assessing the application of the 3Rs: a survey among animal welfare officers in The Netherlands. Laboratory Animals. 47(3): 210–219. doi:10.1177/0023677213483724
28 Chen PJ. (2017) Animal welfare officers in Australian higher education: 3R application, work contexts, and risk perception. Laboratory animals 51(6): 636–646. doi:10.1177/0023677217705152
29 Bourke D, Lindeman M. 2017. Survey of education and training programmes. Proceedings, 2017 Conference, Australian and New Zealand Council for the Care of Animals in Research and Teaching (ANZCCART). Accessed from: https://www.adelaide.edu.au/ANZCCART/publications/proceedings/
30 ORIMA Research. (2018) Survey on the replacement, reduction and refinement of the use of animals for scientific purposes in Australia. Survey Findings Report. Accessed from: https://www.nhmrc.gov.au/research-policy/ethics/animal-ethics/3rs
31 National Centre for the Replacement Refinement and Reduction of Animals in Research (NC3Rs). (2012). Evaluating progress in the 3Rs: the NC3Rs framework. Accessed from: https://www.nc3rs.org.uk/our-evaluation-framework
32 Netherlands National Committee for the protection of animals used for scienti4c purposes (NCad). (2015). Indicators, management and utilisation of data for monitoring laboratory animal use and 3R alternatives, Part 1. Accessed from: https://english.ncadierproevenbeleid.nl/documents/15/11/26/advisory-reports-indicators-management-and-utilisation-of-data-for-monitoring-laboratory-animal-use-and-3r-alternatives
33 Bicknell BA, Pujic Z, Dayan P, Goodhill GJ. (2018) Control of neurite growth and guidance by an inhibitory cell-body signal. PLoS Comput Biol. 14(6): e1006218. doi:10.1371/journal.pcbi.1006218
34 Goodhill G. J. (2018). Theoretical Models of Neural Development. iScience, 8, 183–199. doi:10.1016/j.isci.2018.09.017
35 Horvath A. Organoids: The next revolution in human biology has begun. [Internet]: The University of Melbourne; [accessed 2 April 2019]. Available from: https://pursuit.unimelb.edu.au/articles/organoids-the-next-revolution-in-human-biology-has-begun
36 Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, et al. (2015) Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature.526: 564–568. doi:10.1038/nature17982
37 Little MH. (2016) Growing Kidney Tissue from Stem Cells: How Far from "Party Trick" to Medical Application? Cell Stem Cell. 18(6): 695–8. doi:10.1016/j.stem.2016.05.015
38 Takasato M, Er PX, Chiu HS, Little MH. (2016) Generation of kidney organoids from human pluripotent stem cells. Nature Protocols. 11(9): 1681–92. Epub 2016/08/18. doi:10.1038/nprot.2016.098
39 Tiburcy M, Hudson JE, Balfanz P, Schlick S, Meyer T, Chang Liao M-L, et al. (2017) Defined Engineered Human Myocardium With Advanced Maturation for Applications in Heart Failure Modeling and Repair. Circulation. 135(19): 1832–47. Epub 2017/02/06. doi:10.1161/CIRCULATIONAHA.116.024145
40 Voges HK, Mills RJ, Elliott DA, Parton RG, Porrello ER, Hudson JE. (2017) Development of a human cardiac organoid injury model reveals innate regenerative potential. Development. 144(6): 1118. doi:10.1242/dev.143966
41 Korhonen P, Malm T, White AR. (2018) 3D human brain cell models: New frontiers in disease understanding and drug discovery for neurodegenerative diseases. Neurochem Int. 120: 191–199. Epub 2018 Sep 1. doi:10.1016/j.neuint.2018.08.012
42 Centenera MM, Hickey TE, Jindal S, Ryan NK, Ravindranathan P, Mohammed H, et al. (2018) A patient-derived explant (PDE) model of hormone-dependent cancer. Molecular oncology. 12(9): 1608–22. Epub 2018/08/16. doi:10.1002/1878-0261.12354
43 Wijesundara DK, Ranasinghe C, Jackson RJ, Lidbury BA, Parish CR, Quah BJC. (2014) Use of an In Vivo FTA Assay to Assess the Magnitude, Functional Avidity and Epitope Variant Cross-Reactivity of T Cell Responses Following HIV-1 Recombinant Poxvirus Vaccination. PLoS ONE. 9(8):e105366. doi:10.1371/journal.pone.0105366
44 Carroll CSE, Altin JG, Neeman T, Fahrer AM. (2015) Repeated fine-needle aspiration of solid tumours in mice allows the identification of multiple infiltrating immune cell types. J Immunol Methods. 425: 102–7. doi:10.1016/j.jim.2015.06.015
45 The University of Queensland. Immuno-polymeric drugs for prostate cancer therapy. [Accessed 2 April 2019]. Available from: https://cai.centre.uq.edu.au/project/immuno-polymeric-drugs-prostate-cancer-therapy
46 Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. (2010) Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 8(6):e1000412. doi:10.1371/journal.pbio.1000412
Footnotes
a. The Code applies to the care and use of all live non-human vertebrates and cephalopods. The Code encompasses all aspects of the care and use of animals when the aim is to acquire, develop or demonstrate knowledge or techniques in any area of science – for example, medicine, biology, agriculture, veterinary and other animal sciences, industry and teaching. It includes the use of animals in research, teaching associated with an educational outcome in science, field trials, product testing, diagnosis, the production of biological products and environmental studies.
b. The content of relevant websites and animal ethics application forms may have changed since the completion of the literature review in August 2017.
d. https://www.retsinformation.dk/forms/r0710.aspx?id=162938
e. Competent: the consistent application of knowledge and skill to the standard of performance required regarding the care and use of animals. It embodies the ability to transfer and apply knowledge and skill to new situations and environments.