The Australian Government is now in caretaker period. During this time, updates on this website will be published in accordance with the Guidance on Caretaker Conventions, until after the election.
Professor Frédéric (Fred) Hollande is Deputy Head at the Department of Clinical Pathology, University of Melbourne, and a group leader at the University of Melbourne Centre for Cancer Research. He’s also behind the image recognised in the Science to Art NHMRC Biennial Award.
I came to the University of Melbourne in 2012 from the French National Centre for Scientific Research in Montpellier where I was a senior research fellow and also co-founded a start-up company to enable the translation of some research findings towards clinical applications.
Now at the University of Melbourne, my research group develops innovative and clinically relevant disease models using patient-derived tumour samples, combining phenotypic (functional) and molecular (genomic) analyses of these samples to advance knowledge of complex tumours and to identify novel biomarkers and treatment targets for colorectal and pancreatic cancers.
Over the years, the group has made substantial contributions to the analysis of cell/cell communication and cell signalling during tumour progression.

I have focused on the analysis of mechanisms that underlie the heterogeneity of tumour cells and their resistance to treatment in solid tumours, and published research describing mechanisms regulating the survival and long-term renewal of cancer stem cells, a population of cells that fuel long-term tumour growth.
A strong emphasis of my work has always been to combine important discoveries that drive knowledge acquisition with a strong translational drive, to enable the implementation of personalised oncology and the improvement of therapeutic response.
Multidisciplinary collaborative networks have enabled the generation of high-quality fundamental research data that will lead to clinically relevant translational outcomes, for the benefit of cancer patients.
Tracking and characterizing tumour cells to better target metastatic cancer
Bowel cancer, also known as colorectal cancer, is an interest of mine. This is particularly the case for its metastatic form, due to its devastating impact on patients and their families across the globe.
Metastatic cancers have disseminated from their organ of origin to other areas in the body, and are extremely difficult to comprehensively localise and treat, resulting in very high metastasis-induced mortality.
Bowel cancer most frequently metastasizes to the liver, with lungs and peritoneum representing significant metastasis sites.
While a small proportion of patients demonstrate very long survival after complete surgical removal of metastases, this procedure is impossible in most patients, for whom medical treatment options are very limited. Their metastatic tumours most frequently resist or respond poorly to standard of care chemotherapy regimens. In addition, those with better clinical response always relapse once treatment has ceased.
Our understanding of mechanisms that underpin this poor treatment response of metastases remains very incomplete. Yet, the recent scientific literature points to the fact that different cancer cells within individual tumours can behave differently in many respects, including in terms of their sensitivity to therapeutic drugs. Understanding this concept, called intra-tumour heterogeneity, is essential if we are to efficiently target all metastatic cells and increase the survival of patients with metastatic bowel cancer.
In this context, my team is seeking to better understand the diverse cellular and molecular make-up of liver metastases from patients with metastatic bowel cancer. To do so the team has developed innovative research models, including mini-tumours (organoids) grown in the laboratory from chemotherapy-naïve liver metastases.
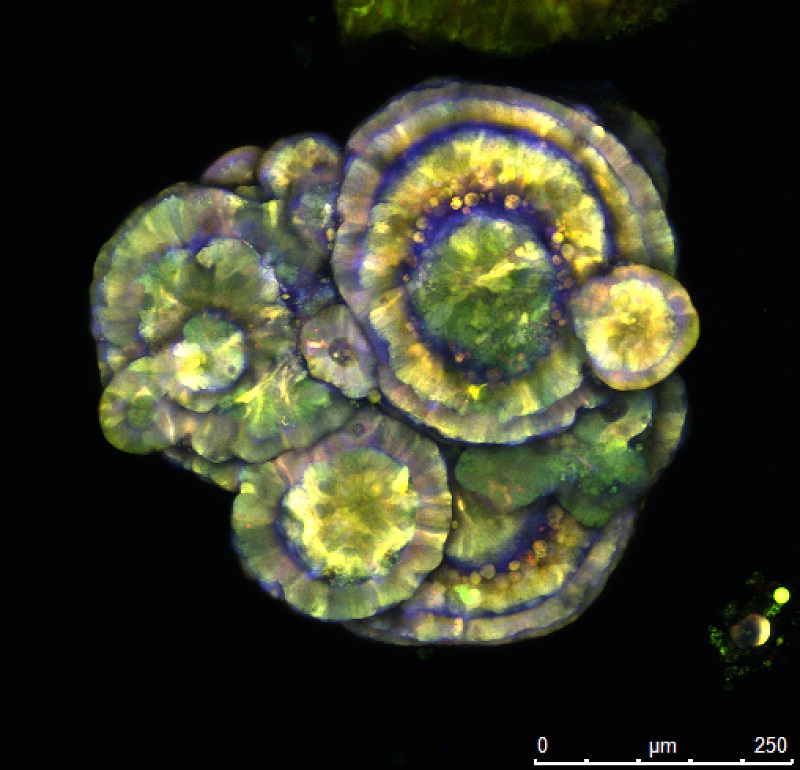
I pioneered a technique named optical barcoding in Australia.
This technique provides a way to distinguish multiple individual tumour cells from one another using fluorescent imaging, and to track all descendants of these different cell subtypes while they are exposed to standard of care drugs, allowing researchers to independently examine the behaviour and molecular response of each cell subtype during and after treatment.
Students and staff in the team have worked hard to develop and implement this technique and other barcoding approaches, aiming to improve our knowledge of the mechanisms that underlie the differential drug sensitivity and recurrence ability that we know exists between various metastatic cells.
In turn, results are leading to the identification and validation of potential treatment alternatives for patients with metastatic bowel cancer, that specifically target processes identified as essential for cells to resist chemotherapy. These novel targeted therapies may then be combined with existing chemotherapeutic drugs to increase the efficacy of patient treatment and to prevent recurrence.

Part of my work is also dedicated to the identification of molecules (biomarkers) that can be used to predict response to these new drugs before treatment has begun, allowing the personalisation of treatment by channelling patients towards the drug they are most likely to respond to.
We really hope that our findings will empower clinicians by guiding their treatment decisions and providing them with new treatment combinations to prolong the survival of bowel cancer patients.
Read more about Professor Hollande's research and the Hollande laboratory: Tumour Heterogeneity in Metastatic Cancer.
